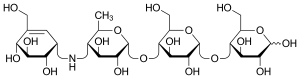
Acarbose
Acarbose (INN)[1] is an anti-diabetic drug used to treat diabetes mellitus type 2 and, in some countries, prediabetes. It is a generic sold in Europe and China as Glucobay (Bayer AG), in North America as Precose (Bayer Pharmaceuticals), and in Canada as Prandase (Bayer AG). It is cheap and popular in China, but not in the U.S. One physician explains the use in the U.S. is limited because it is not potent enough to justify the side effects of diarrhea and flatulence.[2] However, a recent large study concludes "acarbose is effective, safe and well tolerated in a large cohort of Asian patients with type 2 diabetes."[3] A possible explanation for the differing opinions is an observation that acarbose is significantly more effective in patients eating a relatively high carbohydrate Eastern diet.[4]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Glucobay, Precose, Prandase |
| Other names | (2R,3R,4R,5S,6R)-5-{[(2R,3R,4R,5S,6R)-5- {[(2R,3R,4S,5S,6R)-3,4-dihydroxy-6-methyl- 5-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3- (hydroxymethyl)cyclohex-2-en-1-yl]amino} tetrahydro-2H-pyran-2-yl]oxy}-3,4-dihydroxy- 6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}- 6-(hydroxymethyl)tetrahydro-2H-pyran-2,3,4-triol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696015 |
| License data | |
| Pregnancy category | |
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Extremely low |
| Metabolism | Gastrointestinal tract |
| Elimination half-life | 2 hours |
| Excretion | Renal (less than 2%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.054.555 |
| Chemical and physical data | |
| Formula | C25H43NO18 |
| Molar mass | 645.608 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
It is a starch blocker, and inhibits alpha glucosidase, an intestinal enzyme that releases glucose from larger carbohydrates. It is composed of an acarviosin moiety with a maltose at the reducing terminus.
Mechanism of action
Acarbose inhibits enzymes (glycoside hydrolases) needed to digest carbohydrates, specifically, alpha-glucosidase enzymes in the brush border of the small intestines, and pancreatic alpha-amylase. Pancreatic alpha-amylase hydrolyzes complex starches to oligosaccharides in the lumen of the small intestine, whereas the membrane-bound intestinal alpha-glucosidases hydrolyze oligosaccharides, trisaccharides, and disaccharides to glucose and other monosaccharides in the small intestine. Inhibition of these enzyme systems reduces the rate of digestion of complex carbohydrates. Less glucose is absorbed because the carbohydrates are not broken down into glucose molecules. In diabetic patients, the short-term effect of these drug therapies is to decrease current blood glucose levels; the long-term effect is a reduction in HbA1c level.[5] This reduction averages an absolute decrease of 0.7%, which is a decrease of about 10% in typical HbA1c values in diabetes studies.[6]
Combination therapy
The combination of acarbose with metformin results in greater reductions of HbA1c, fasting blood glucose and post-prandial glucose than either agent alone.[7]
Dosing
Since acarbose prevents the digestion of complex carbohydrates, the drug should be taken at the start of main meals (taken with first bite of meal). Moreover, the amount of complex carbohydrates in the meal will determine the effectiveness of acarbose in decreasing postprandial hyperglycemia. Adults may take doses of 25 mg 3 times daily, increasing to 100 mg 3 times a day. However the maximum dose per day is 600 mg.
Side-effects
Since acarbose prevents the degradation of complex carbohydrates into glucose, some carbohydrate will remain in the intestine and be delivered to the colon. In the colon, bacteria digest the complex carbohydrates, causing gastrointestinal side-effects such as flatulence (78% of patients) and diarrhea (14% of patients). Since these effects are dose-related, in general it is advised to start with a low dose and gradually increase the dose to the desired amount. One study found that gastrointestinal side effects decreased significantly (from 50% to 15%) over 24 weeks, even on constant dosing.[8]
If a patient using acarbose suffers from a bout of hypoglycemia, the patient must eat something containing monosaccharides, such as glucose tablets or gel (GlucoBurst, Insta-Glucose, Glutose, Level One) and a doctor should be called. Because acarbose blocks the breakdown of table sugar and other complex sugars, fruit juice or starchy foods will not effectively reverse a hypoglycemic episode in a patient taking acarbose.[9]
Hepatitis has been reported with acarbose use. It usually goes away when the medicine is stopped.[10] Therefore, liver enzymes should be checked before and during use of this medicine.
Life extension
In studies conducted by three independent laboratories by the US National Institute on Aging's intervention testing programme, acarbose was shown to extend the lifespan of female mice by 5% and of male mice by 22%.[11][12]
References
- "International Nonproprietary Names for Pharmaceutical Substances. Recommended International Nonproprietary Names (Rec. INN): List 19" (PDF). World Health Organization. 1979. Retrieved 9 November 2016.
- Kresge N (21 November 2011). "China's Thirst for New Diabetes Drugs Threatens Bayer's Lead". Bloomberg Business Week. Archived from the original on 21 November 2011. Retrieved 15 April 2016.
- Zhang W, Kim D, Philip E, Miyan Z, Barykina I, Schmidt B, Stein H (April 2013). "A multinational, observational study to investigate the efficacy, safety and tolerability of acarbose as add-on or monotherapy in a range of patients: the Gluco VIP study". Clinical Drug Investigation. 33 (4): 263–74. doi:10.1007/s40261-013-0063-3. PMID 23435929. S2CID 207483590.
- Zhu Q, Tong Y, Wu T, Li J, Tong N (June 2013). "Comparison of the hypoglycemic effect of acarbose monotherapy in patients with type 2 diabetes mellitus consuming an Eastern or Western diet: a systematic meta-analysis". Clinical Therapeutics. 35 (6): 880–99. doi:10.1016/j.clinthera.2013.03.020. PMID 23602502.
- Drug Therapy in Nursing, 2nd Edition.
- Scheen AJ (September 1998). "Clinical efficacy of acarbose in diabetes mellitus: a critical review of controlled trials". Diabetes & Metabolism. 24 (4): 311–20. PMID 9805641.
- Hedrington MS, Davis SN (2019). "Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes". Expert Opinion on Pharmacotherapy. 20 (18): 2229–2235. doi:10.1080/14656566.2019.1672660. PMID 31593486.
- Hoffmann J, Spengler M (December 1997). "Efficacy of 24-week monotherapy with acarbose, metformin, or placebo in dietary-treated NIDDM patients: the Essen-II Study". The American Journal of Medicine. 103 (6): 483–90. doi:10.1016/S0002-9343(97)00252-0. PMID 9428831.
- "Acarbose". MedlinePlus Drug Information.
- "Acarbose: hepatitis: France, Spain". WHO Pharmaceuticals Newsletter. 1999.
- Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, Nelson JF, Pletcher S, Simpkins JW, Smith D, Wilkinson JE, Miller RA (2014). "Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males". Aging Cell. 13 (2): 273–282. doi:10.1111/acel.12170. PMC 3954939. PMID 24245565.
- Ladiges W, Liggitt D (2017). "Testing drug combinations to slow aging". Pathobiology of Aging & Age-related Diseases. 8 (1): 1407203. doi:10.1080/20010001.2017.1407203. PMC 5706479. PMID 29291036.
External links
- "Acarbose". Drug Information Portal. U.S. National Library of Medicine.
- "Probing the Pancreas" - by Craig D. Reid, Ph.D. (US FDA Consumer Article)