Cellobiose
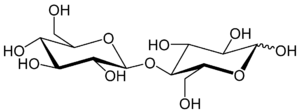
Cellobiose is a disaccharide with the formula (C6H7(OH)4O)2O. It is classified as a reducing sugar. In terms of its chemical structure, it is derived from the condensation of a pair β-glucose molecules forging a β(1→4) bond. It can be hydrolyzed to glucose enzymatically or with acid. Cellobiose has eight free alcohol (OH) groups, one acetal linkage and one hemiacetal linkage, which give rise to strong inter- and intramolecular hydrogen bonds. It is a white solid.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.670 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H22O11 | |
| Molar mass | 342.297 g·mol−1 |
| Appearance | white, hard powder |
| Odor | odorless |
| Density | 1.768 g/mL |
| Melting point | 203.5 °C (398.3 °F; 476.6 K) (decomposes) |
| 12 g/100mL | |
| Solubility | very slightly soluble in alcohol insoluble in ether, chloroform |
| log P | -5.03 |
| Acidity (pKa) | 12.39 |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It can be obtained by enzymatic or acidic hydrolysis of cellulose and cellulose-rich materials such as cotton, jute, or paper.[1] Cellobiose can be used as an indicator carbohydrate for Crohn's disease and malabsorption syndrome.[2]
Treatment of cellulose with acetic anhydride and sulfuric acid, gives cellobiose octoacetate, which is no longer a hydrogen bond donor (though it is still a hydrogen bond acceptor) and is soluble in nonpolar organic solvents.[3]
References
- Wilson, David B. (2009). "Cellulases and biofuels". Current Opinion in Biotechnology. 20 (3): 295–299. doi:10.1016/j.copbio.2009.05.007. PMID 19502046.
- Braun, G. (1943). "α-Cellobiose Octaacetate" (PDF). Organic Syntheses. Collected Volume 2: 124. and Braun, G. (1937). "α-Cellobiose Octaacetate". Organic Syntheses. 17: 36. doi:10.15227/orgsyn.017.0036.
