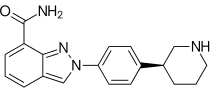
Niraparib
Niraparib (trade name Zejula) is an orally active[1] small molecule PARP inhibitor developed by Tesaro to treat ovarian cancer.
 | |
| Clinical data | |
|---|---|
| Trade names | Zejula |
| Other names | MK-4827 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a617007 |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 73% |
| Protein binding | 83% |
| Metabolism | Carboxylesterases |
| Metabolites | M1 (carboxylic acid) |
| Elimination half-life | 36 hours |
| Excretion | 48% urine, 29% feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.210.548 |
| Chemical and physical data | |
| Formula | C19H20N4O |
| Molar mass | 320.396 g·mol−1 |
| 3D model (JSmol) | |
| Solubility in water | 0.7–1.1 mg/mL (20 °C) |
| |
| |
Niraparib was granted fast track designation by the US Food and Drug Administration (FDA), and Tesaro submitted a new drug application in 2016.[2] It was approved on 27 March 2017 in the US,[3] and has been approved in Europe on 16 November 2017.[4]
Medical uses
The drug is approved by the US FDA for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in complete or partial response to platinum-based chemotherapy.[3]
In a study with 553 patients, progression-free survival (PFS) for patients with a deleterious or suspected deleterious BRCA mutation in the germline was 21.0 months under niraparib therapy, as compared to 5.5 months under placebo. Patients without such a mutation had a PFS of 9.3 months under niraparib versus 3.9 months under placebo.[3][5]
Contraindications
No contraindications are listed in the prescribing information.[6]
Side effects
The most common side effects in studies were low blood cell counts, namely thrombocytopenia (in 61% of patients, severe in 29%), anemia (in 50%, severe in 25%) and neutropenia (in 30%, severe in 20%). Other, mostly mild to moderate side effects included nausea, fatigue, and constipation. In a study running over 250 days (median), 15% of patients had to permanently discontinue niraparib due to adverse effects.[6]
Interactions
No clinical interaction studies have been performed. The potential for interactions with other drugs is low as niraparib and its main metabolite M1 do not significantly interact with any of the important cytochrome P450 liver enzymes in vitro. Niraparib, but not M1, is transported by P-glycoprotein and BCRP, but does not significantly inhibit them. Neither niraparib nor M1 significantly interact with any of the other important transporter proteins.[6]
Pharmacology
Pharmacokinetics
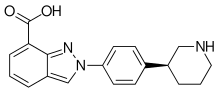
73% of ingested niraparib is absorbed in the gut,[9] and it reaches highest blood plasma concentrations after about three hours, independently of food intake. In the circulation, 83% of the substance are bound to plasma proteins. It is inactivated by carboxylesterases to the main metabolite M1, the carboxylic acid derivative,[8] which is subsequently glucuronidated.[6]
The mean biological half-life is 36 hours. 47.5% of the substance are found in the urine and 38.8% in the feces. Unmetabolised niraparib accounts for 11% in the urine and 19% in the feces.[8]
Chemistry
The drug is used in form of the salt niraparib tosylate monohydrate, which is white to off-white, non-hygroscopic crystals.[6]
Studies
A 2012 study in a cell line found that PARP inhibitors exhibit cytotoxic effects not based solely on their enzymatic inhibition of PARP, but by their trapping of PARP on damaged DNA, and the strength of this trapping activity was ordered niraparib >> olaparib >> veliparib.[10]
References
- Clinical trial number NCT01905592 for "A Phase III Trial of Niraparib Versus Physician's Choice in HER2 Negative, Germline BRCA Mutation-positive Breast Cancer Patients (BRAVO)" at ClinicalTrials.gov
- "Niraparib Receives FDA Fast Track Designation for the Treatment of Recurrent Platinum-Sensitive Ovarian, Fallopian Tube, or Primary Peritoneal Cancer". The European Society for Medical Oncology (ESMO). 5 September 2016.
- "Niraparib (Zejula)". US FDA. 30 March 2017.
- "Zejula". European Medicines Agency. 17 September 2018.
- Adams B (29 June 2016). "Tesaro's PARP ovarian cancer drug hits PhIII goal; prepares to file". Fierce Biotech.
- Zejula FDA Professional Drug Information.
- "PARP inhibitor, MK-4827, shows anti-tumor activity in first trial in humans". 17 November 2010.
- van Andel L, Zhang Z, Lu S, Kansra V, Agarwal S, Hughes L, et al. (December 2017). "14C-niraparib, a novel poly(ADP-Ribose) polymerase (PARP)-1 and PARP-2 inhibitor, in patients with advanced cancer". Investigational New Drugs. 35 (6): 751–765. doi:10.1007/s10637-017-0451-2. PMC 5694528. PMID 28303528.
- van Andel L, Rosing H, Zhang Z, Hughes L, Kansra V, Sanghvi M, et al. (January 2018). "14C-microtracer and therapeutic dose in cancer patients". Cancer Chemotherapy and Pharmacology. 81 (1): 39–46. doi:10.1007/s00280-017-3455-x. PMC 5754411. PMID 29043410.
- Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. (November 2012). "Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors". Cancer Research. 72 (21): 5588–99. doi:10.1158/0008-5472.CAN-12-2753. PMC 3528345. PMID 23118055.
External links
- "Niraparib". Drug Information Portal. U.S. National Library of Medicine.