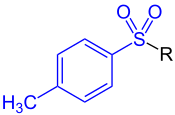
Tosyl
A toluenesulfonyl (shortened tosyl, abbreviated Ts or Tos) group is CH3C6H4SO2. This group is usually derived from the compound tosyl chloride, CH3C6H4SO2Cl (abbreviated TsCl), which forms esters and amides of toluenesulfonic acid. The para orientation illustrated (p-toluenesulfonyl) is most common, and by convention tosyl refers to the p-toluenesulfonyl group.
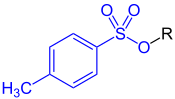
Tosylate refers to the anion of p-toluenesulfonic acid (CH
3C
6H
4SO−
3) and it is abbreviated as TsO−, or it may refer to esters of p-toluenesulfonic acid.
Applications
For SN2 reactions, alkyl alcohols can also be converted to alkyl tosylates, often through addition of tosyl chloride. In this reaction, the lone pair of the alcohol oxygen attacks the sulfur of the tosyl chloride, displacing the chloride and forming the tosylate with retention of reactant stereochemistry. This is useful because alcohols are poor leaving groups in SN2 reactions, in contrast to the tosylate group. It is the transformation of alkyl alcohols to alkyl tosylates that allows an SN2 reaction to occur in the presence of a good nucleophile.
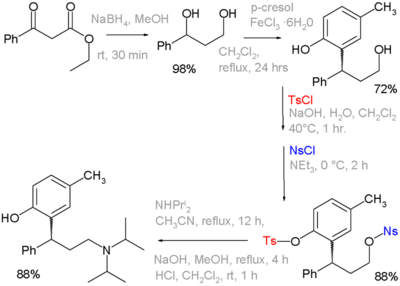
A tosyl group can function as a protecting group in organic synthesis. Alcohols can be converted to tosylate groups so that they do not react. The tosylate group may later be converted back into an alcohol. The use of these functional groups is exemplified in organic synthesis of the drug tolterodine, wherein one of the steps a phenol group is protected as its tosylate and the primary alcohol as its nosylate. The latter is a leaving group for displacement by diisopropylamine:[1][nb 1]
The tosyl group is also useful as a protecting group for amines. The resulting sulfonamide structure is extremely stable. It can be deprotected to reveal the amine using reductive or strongly acidic conditions.[2]
Amine protection – tosyl (Ts)

Tosyl (Ts) group is commonly used as a protecting group for amines in organic synthesis.
Most common amine protection methods
- Tosyl chloride and pyridine in dichloromethane[3]
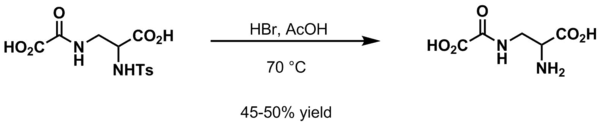
Most common amine deprotection methods
- HBr and acetic acid at 70 °C[4]
- Refluxing with TMSCl, sodium iodide and acetonitrile[5]
Related compounds
Closely related to the tosylates are the nosylates and brosylates. There are p-nitrobenzenesulfonates and p-bromobenzenesulfonates, respectively.
See also
- Tosylic acid
- Sulfonyl group
Notes
- Reaction sequence: organic reduction of ethyl benzoylacetate by sodium borohydride to a diol, followed by Friedel-Crafts alkylation with p-cresol and iron(III) chloride to a phenol. The eat and nosyl groups are introduced as their respective chlorides with either sodium hydroxide or triethylamine as a base. The next step is nucleophilic displacement of the nosyl group by diisopropylamine, the remaining tosyl group is removed by another round of NaOH. Not shown: optical resolution by L-tartaric acid to optically pure (R)-isomer
References
- Kathlia A. De Castro; Jungnam Ko; Daejong Park; Sungdae Park & Hakjune Rhee (2007). "Reduction of Ethyl Benzoylacetate and Selective Protection of 2-(3-Hydroxy-1-phenylpropyl)-4-methylphenol: A New and Facile Synthesis of Tolterodine". Organic Process Research & Development (ASAP article)
|format=requires|url=(help). 11 (5): 918. doi:10.1021/op7001134. - Greene, T. W.; Wuts, P. G. M. (1999). Protective Groups In Organic Synthesis. New York: John Wiley & Sons. pp. 603–607. ISBN 9780471160199.
- Wuts, Peter G. M.; Greene, Theodora W. (2006). Greene's Protective Groups in Organic Synthesis, Fourth Edition - Wuts - Wiley Online Library. doi:10.1002/0470053488. ISBN 9780470053485.
- Haskell, Betty E.; Bowlus, Stephen B. (1976-01-01). "New synthesis of L-2-amino-3-oxalylaminopropionic acid, the Lathyrus sativus neurotoxin". The Journal of Organic Chemistry. 41 (1): 159–160. doi:10.1021/jo00863a042. ISSN 0022-3263. PMID 1244456.
- Sabitha, Gowravaram; Reddy, B. V. Subba; Abraham, Sunny; Yadav, J. S. (1999-02-19). "Deprotection of sulfonamides using iodotrimethylsilane". Tetrahedron Letters. 40 (8): 1569–1570. doi:10.1016/S0040-4039(98)02646-X.