Water treatment
Water treatment is any process that improves the quality of water to make it appropriate for a specific end-use. The end use may be drinking, industrial water supply, irrigation, river flow maintenance, water recreation or many other uses, including being safely returned to the environment. Water treatment removes contaminants and undesirable components, or reduces their concentration so that the water becomes fit for its desired end-use. This treatment is crucial to human health and allows humans to benefit from both drinking and irrigation use.

Drinking water treatment
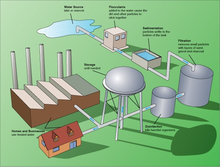
Treatment for drinking water production involves the removal of contaminants from raw water to produce water that is pure enough for human consumption without any short term or long term risk of any adverse health effect. In general terms, the greatest microbial risks are associated with ingestion of water that is contaminated with human or animal (including bird) faeces. Faeces can be a source of pathogenic bacteria, viruses, protozoa and helminths. The destruction of microbial pathogens is essential and very commonly involves the use of reactive chemical agents such suspended solids, bacteria, algae, viruses, fungi, and minerals such as iron and manganese. These substances continue to cause great harm to several lower developed countries who do not have access to water purification.
Measures taken to ensure water quality not only relate to the treatment of the water, but to its conveyance and distribution after treatment. It is therefore common practice to keep residual disinfectants in the treated water to kill bacteriological contamination during distribution.
Water supplied to domestic properties, for tap water or other uses, may be further treated before use, often using an in-line treatment process. Such treatments can include water softening or ion exchange. Many proprietary systems also claim to remove residual disinfectants and heavy metal ions.
Processes

The processes involved in removing the contaminants include physical processes such as settling and filtration, chemical processes such as disinfection and coagulation, and biological processes such as slow sand filtration.
A combination selected from the following processes is used for municipal drinking water treatment worldwide.
Chemical

- Pre-chlorination for algae control and arresting biological growth.
- Aeration along with pre-chlorination for removal of dissolved iron when present with small amounts relatively of manganese.
- Disinfection for killing bacteria, viruses and other pathogens, using chlorine, ozone and ultra-violet light.
Physical
- Sedimentation for solids separation that is the removal of suspended solids trapped in the floc.
- Filtration to remove particles from water either by passage through a sand bed that can be washed and reused or by passage through a purpose designed filter that may be washable.
- Dissolved air flotation to remove suspended solids.
Physio-chemical
- Coagulation for flocculation.
- Coagulant aids, also known as polyelectrolytes – to improve coagulation and for more robust floc formation.
Biological
- Slow sand filtration using a biofilm to metabolize organic matter.
Technologies
Technologies for potable water and other uses are well-developed, and generalized designs are available from which treatment processes can be selected for pilot testing on the specific source water. In addition, a number of private companies provide patented technological solutions for the treatment of specific contaminants. Automation of water treatment is common in the developed world. Source water quality through the seasons, scale, and environmental impact can dictate capital costs and operating costs. End use of the treated water dictates the necessary quality monitoring technologies, and locally available skills typically dictate the level of automation adopted.
Distillation
Saline water can be treated to yield fresh water. Two main processes are used, reverse osmosis or distillation.[1] Both methods require more energy than water treatment of local surface waters, and are usually only used in coastal areas or where water such as groundwater has high salinity.[2][3]
Portable Water Purification
Living away from drinking water supplies often requires some form of portable water treatment process. These can vary in complexity from the simple addition of a disinfectant tablet in a hiker's water bottle through to complex multi-stage processes carried by boat or plane to disaster areas.
| Constituent | Unit Processes |
| Turbidity and particles | Coagulation/ flocculation, sedimentation, granular filtration |
| Major dissolved inorganics | Softening, aeration, membranes |
| Minor dissolved inorganics | Membranes |
| Pathogens | Sedimentation, filtration, disinfection |
| Major dissolved organics | Membranes, adsorption |
Standards
Many developed countries specify standards to be applied in their own country. In Europe, this includes the European Drinking Water Directive[4] and in the United States the United States Environmental Protection Agency (EPA) establishes standards as required by the Safe Drinking Water Act. For countries without a legislative or administrative framework for such standards, the World Health Organization publishes guidelines on the standards that should be achieved.[5] China adopted its own drinking water standard GB3838-2002 (Type II) enacted by Ministry of Environmental Protection in 2002.[6]
Where drinking water quality standards do exist, most are expressed as guidelines or targets rather than requirements, and very few water standards have any legal basis or, are subject to enforcement.[7] Two exceptions are the European Drinking Water Directive and the Safe Drinking Water Act in the United States, which require legal compliance with specific standards.
Industrial water treatment
Processes
Two of the main processes of industrial water treatment are boiler water treatment and cooling water treatment. A large amount of proper water treatment can lead to the reaction of solids and bacteria within pipe work and boiler housing. Steam boilers can suffer from scale or corrosion when left untreated. Scale deposits can lead to weak and dangerous machinery, while additional fuel is required to heat the same level of water because of the rise in thermal resistance. Poor quality dirty water can become a breeding ground for bacteria such as Legionella causing a risk to public health.
Corrosion in low pressure boilers can be caused by dissolved oxygen, acidity and excessive alkalinity. Water treatment therefore should remove the dissolved oxygen and maintain the boiler water with the appropriate pH and alkalinity levels. Without effective water treatment, a cooling water system can suffer from scale formation, corrosion and fouling and may become a breeding ground for harmful bacteria. This reduces efficiency, shortens plant life and makes operations unreliable and unsafe.[8]
Boiler water treatment
Boiler water treatment is a type of industrial water treatment focused on removal or chemical modification of substances potentially damaging to the boiler. Varying types of treatment are used at different locations to avoid scale, corrosion, or foaming. External treatment of raw water supplies intended for use within a boiler is focused on removal of impurities before they reach the boiler. Internal treatment within the boiler is focused on limiting the tendency of water to dissolve the boiler, and maintaining impurities in forms least likely to cause trouble before they can be removed from the boiler in boiler blowdown.
Cooling water treatment
Water cooling is a method of heat removal from components and industrial equipment. Water may be a more efficient heat transfer fluid where air cooling is ineffective. In most occupied climates water offers the thermal conductivity advantages of a liquid with unusually high specific heat capacity and the option of evaporative cooling. Low cost often allows rejection as waste after a single use, but recycling coolant loops may be pressurized to eliminate evaporative loss and offer greater portability and improved cleanliness. Unpressurized recycling coolant loops using evaporative cooling require a blowdown waste stream to remove impurities concentrated by evaporation. Disadvantages of water cooling systems include accelerated corrosion and maintenance requirements to prevent heat transfer reductions from biofouling or scale formation. Chemical additives to reduce these disadvantages may introduce toxicity to wastewater. Water cooling is commonly used for cooling automobile internal combustion engines and large industrial facilities such as nuclear and steam electric power plants, hydroelectric generators, petroleum refineries and chemical plants.
Technologies
Chemical treatment
Chemical treatments are techniques adopted to make industrial water suitable for use or discharge. These include chemical precipitation, chemical disinfection, chemical oxidation, advanced oxidation, ion exchange, and chemical neutralization.[9]
Physical treatment
Filtration removes particles from water either by passage through a layer of sand, such as a rapid gravity filter,[10] or in a mechanical filter.
Dissolved air flotation removes suspended solids from the water.[11] This is achieved by dissolving air in the water under pressure and then releasing the water/air at atmospheric pressure in a flotation tank. The released air forms small bubbles which adhere to the suspended matter causing them to float to the surface of the water where they can be removed by a skimming device or an overflow.[11]
Biological treatment
Slow sand filters use a biological process to purify raw water to produce potable water.[12] They work by using a complex biological film that grows naturally on the surface of sand. This gelatinous biofilm called the hypogeal layer or Schmutzdecke is located in the upper few millimetres of the sand layer. The surface biofilm purifies the water as it flows through the layer, the underlying sand provides a support medium for the biological treatment layer.[13] The Schmutzdecke consists of bacteria, fungi, protozoa, rotifera and a range of aquatic insect larvae. As the biofilm ages, more algae may develop and larger aquatic organisms including bryozoa, snails and Annelid worms may be present. As water passes through the hypogeal layer, particles of matter are trapped in the mucilaginous matrix and soluble organic material is adsorbed. The contaminants are metabolised by the bacteria, fungi and protozoa.[12]
Slow sand filters are typically 1 – 2 metres deep, and have a hydraulic loading rate of 0.2 – 0.4 cubic metres per square metre per hour.[13] Filters lose their performance as the biofilm thickens and reduces the rate of flow. The filter is refurbished by removing the biofilm and a thin upper layer of sand. Water is decanted back into the filter and re-circulated to enable a new biofilm to develop. Alternatively wet harrowing involves stirring the sand and flushing the biolayer through for disposal.[13]
Physio-chemical treatment
Chemical flocculants are used to generate a floc in the water that traps suspended solids. Chemical polyelectrolytes are used to increase coagulation of suspended solids to improve removal.[14]
Developing countries
Appropriate technology options in water treatment include both community-scale and household-scale point-of-use (POU) or self-supply designs.[15] Such designs may employ solar water disinfection methods, using solar irradiation to inactivate harmful waterborne microorganisms directly, mainly by the UV-A component of the solar spectrum, or indirectly through the presence of an oxide photocatalyst, typically supported TiO2 in its anatase or rutile phases.[16] Despite progress in SODIS technology, military surplus water treatment units like the ERDLator are still frequently used in developing countries. Newer military style Reverse Osmosis Water Purification Units (ROWPU) are portable, self-contained water treatment plants are becoming more available for public use.[17]
For waterborne disease reduction to last, water treatment programs that research and development groups start in developing countries must be sustainable by the citizens of those countries. This can ensure the efficiency of such programs after the departure of the research team, as monitoring is difficult because of the remoteness of many locations.
Energy Consumption: Water treatment plants can be significant consumers of energy. In California, more than 4% of the state's electricity consumption goes towards transporting moderate quality water over long distances, treating that water to a high standard.[18] In areas with high quality water sources which flow by gravity to the point of consumption, costs will be much lower. Much of the energy requirements are in pumping. Processes that avoid the need for pumping tend to have overall low energy demands. Those water treatment technologies that have very low energy requirements including trickling filters, slow sand filters, gravity aqueducts.
Regulation
United States
The Safe Drinking Water Act requires the U.S. Environmental Protection Agency (EPA) to set standards for drinking water quality in public water systems (entities that provide water for human consumption to at least 25 people for at least 60 days a year).[19] Enforcement of the standards is mostly carried out by state health agencies.[20] States may set standards that are more stringent than the federal standards.[21]
EPA has set standards for over 90 contaminants organized into six groups: microorganisms, disinfectants, disinfection byproducts, inorganic chemicals, organic chemicals and radionuclides.[22]
EPA also identifies and lists unregulated contaminants which may require regulation. The Contaminant Candidate List is published every five years, and EPA is required to decide whether to regulate at least five or more listed contaminants.[23]
Local drinking water utilities may apply for low interest loans, to make facility improvements, through the Drinking Water State Revolving Fund.[24]
United Kingdom
In the United Kingdom regulation of water supplies is a devolved matter to the Welsh and Scottish Parliaments and the Northern Ireland Assembly.
In England and Wales there are two water industry regulatory authorities.
- Water Services Regulation Authority (Ofwat) is the economic regulator of the water sector; it protects the interests of consumers by promoting effective competition and ensuring that water companies carry out their statutory functions. Ofwat has a management Board comprising a Chairman, Chief Executive and Executive and Non-Executive members. There is a staff of about 240.[25]
- The Drinking Water Inspectorate (DWI) provides independent assurance that the privatised water industry delivers safe, clean drinking water to consumers. The DWI was established in 1990 and comprises a Chief Inspector of Drinking Water and a team of about 40 people.[26] The current standards of water quality are defined in Statutory Instrument 2016 No. 614 the Water Supply (Water Quality) Regulations 2016.[27]
The functions and duties of the bodies are formally defined in the Water Industry Act 1991 (1991 c. 56)[28] as amended by the Water Act 2003 (2003 c. 37) and the Water Act 2014 (2014 c. 21).
In Scotland water quality is the responsibility of independent Drinking Water Quality Regulator (DWQR).[29]
In Northern Ireland the Drinking Water Inspectorate (DWI) regulates drinking water quality of public and private supplies.[30] The current standards of water quality are defined in the Water Supply (Water Quality) Regulations (Northern Ireland) 2017.[31]
See also
References
- "Water Desalination". Stanford University. 16 December 2002. Retrieved 29 October 2019.
- Lienhard, John H.; Thiel, Gregory P.; Warsinger, David M.; Banchik, Leonardo D. (2016-12-08). "Low Carbon Desalination: Status and Research, Development, and Demonstration Needs, Report of a workshop conducted at the Massachusetts Institute of Technology in association with the Global Clean Water Desalination Alliance". Massachusetts Institute of Technology. hdl:1721.1/105755. Cite journal requires
|journal=(help) - Rouzafay, F.; Shidpour, R. (2020). "Lifetime and dynamics of charge carriers in carbon-incorporated ZnO nanostructures for water treatment under visible light: Femtosecond transient absorption and photoluminescence study". Environmental Chemical Engineering. 8. doi:10.1016/j.jece.2020.104097.
- European Drinking Water Directive
- Guidelines for Drinking-water Quality, Fourth Edition; World Health Organization; 2011
- "Environmental quality standards for surface water".
- What is the purpose of drinking water quality guidelines/regulations?. Canada: Safe Drinking Water Foundation. Pdf. Archived 2011-10-06 at the Wayback Machine
- Cicek, V. (2013). "Corrosion and corrosion prevention in boilers". Cathodic protection: industrial solutions for protecting against corrosion. Hoboken, New Jersey: John Wiley & Sons. ISBN 9781118737880.
- Pal, Parimal (2017-01-01), Pal, Parimal (ed.), "Chapter 2 - Chemical Treatment Technology", Industrial Water Treatment Process Technology, Butterworth-Heinemann, pp. 21–63, ISBN 9780128103913, retrieved 2019-11-19
- "Rapid Gravity Filter". Science Direct. 2009. Retrieved 26 June 2020.
- Wong, Joe (2013). "Dissolved Air Flotation". Water World. Retrieved 26 June 2020.
- SSWM University. "Slow sand filtration". SSWM University. Retrieved 26 June 2020.
- B. Sizirici Yildiz (2012). "Slow sand filtration". Science Direct. Retrieved 26 June 2020.
- SSWM University. "Coagulation - Flocculation". SSWM University. Retrieved 26 June 2020.
- "Household Water Treatment Guide". Centre for Affordable Water and Sanitation Technology, Canada. March 2008.
- "Sand as a low-cost support for titanium dioxide photocatalysts". Materials Views. Wiley VCH.
- Lindsten, Don C. (September 1984). "Technology transfer: Water purification, U.S. Army to the civilian community". The Journal of Technology Transfer. 9 (1): 57–59. doi:10.1007/BF02189057.
- "Energy Costs of Water in California". large.stanford.edu. Retrieved 2017-05-07.
- United States. Safe Drinking Water Act. Pub.L. 93–523; 88 Stat. 1660; 42 U.S.C. § 300f et seq. 1974-12-16.
- "Primacy Enforcement Responsibility for Public Water Systems". Drinking Water Requirements for States and Public Water Systems. Washington, D.C.: United States Environmental Protection Agency (EPA). 2016-11-02.
- Understanding the Safe Drinking Water Act (Report). EPA. June 2004. EPA 816-F-04-030.
- "National Primary Drinking Water Regulations". Ground Water and Drinking Water. EPA. 2019-09-17.
- "Basic Information on the CCL and Regulatory Determination". Contaminant Candidate List. EPA. 2019-07-19.
- "Drinking Water State Revolving Fund". EPA. 2019-10-30.
- "Ofwat". Ofwat.gov.uk. Retrieved 10 July 2020.
- "Drinking Water Inspectorate". dwi.gov.uk. Retrieved 10 July 2020.
- "Water Supply (Water Quality) Regulations 2016". legislation.gov.uk. Retrieved 10 July 2020.
- "Water Industry Act 1991". legislation.gov.uk. Retrieved 10 July 2020.
- "Water Quality Regulator". Scottish Government. Retrieved 10 July 2020.
- "Drinking Water Inspectorate". daera-ni.gov.uk. Retrieved 10 July 2020.
- "Water Supply (Water Quality) Regulations (Northern Ireland) 2017". legislation.gov.uk.
Further reading
- Eaton, Andrew D.; Franson, Mary Ann H. (2005). Standard methods for the examination of water and wastewater (21 ed.). American Public Health Association. ISBN 978-0-87553-047-5.
External links
| Wikimedia Commons has media related to Water treatment. |
- International Water Association Professional / research organization
- Center for Biological and Environmental Nanotechnology (CBEN), Rice University
- NSF International – Independent non-profit standards organization
- WHO.int, WHO Guidelines
- Safe and Sustainable Water for Haiti web site hosted by Grand Valley State University