Tellurium tetrabromide
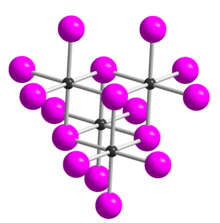
Tellurium tetrabromide (TeBr4) is an inorganic chemical compound. It has a similar tetrameric structure to TeCl4.[2] It can be made by reacting bromine and tellurium.[3] In the vapour TeBr4 dissociates:[2]
- TeBr4 → TeBr2 + Br2
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.070 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| TeBr4 | |
| Molar mass | 447.22 g/mol |
| Appearance | yellow-orange crystals |
| Density | 4.3 g/cm3, solid |
| Melting point | 388 °C (730 °F; 661 K)[1] |
| Boiling point | decomposes at 420 °C (788 °F; 693 K) |
| Structure | |
| monoclinic | |
| Hazards | |
EU classification (DSD) (outdated) |
not listed |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is a conductor when molten, dissociating into the ions TeBr3+ and Br− When dissolved in benzene and toluene, TeBr4 is present as the unionized tetramer Te4Br16.[2] In solvents with donor properties such as acetonitrile, CH3CN ionic complexes are formed which make the solution conducting:
- TeBr4 + 2CH3CN → (CH3CN)2TeBr3+ + Br−
References
- Thermochemical Data of Elements and Compounds", M. Binnewies, E. Milke, Wiley-VCH, 2002, ISBN 3-527-30524-6
- Inorganic Chemistry,Egon Wiberg, Arnold Frederick Holleman Elsevier 2001 ISBN 0-12-352651-5
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.