tRNA isopentenyltransferase
In enzymology, a tRNA isopentenyltransferase (EC 2.5.1.8) is an enzyme that catalyzes the chemical reaction
- isopentenyl diphosphate + tRNA diphosphate + tRNA containing 6-isopentenyladenosine
| tRNA isopentenyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 2.5.1.8 | ||||||||
| CAS number | 37277-78-4 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Thus, the two substrates of this enzyme are isopentenyl diphosphate and tRNA, whereas its two products are diphosphate and tRNA containing 6-isopentenyladenosine.
This enzyme belongs to the family of transferases, specifically those transferring aryl or alkyl groups other than methyl groups. The systematic name of this enzyme class is isopentenyl-diphosphate:tRNA isopentenyltransferase. Other names in common use include transfer ribonucleate isopentenyltransferase, Delta2-isopentenyl pyrophosphate:tRNA-Delta2-isopentenyl, transferase, Delta2-isopentenyl pyrophosphate:transfer ribonucleic acid, and Delta2-isopentenyltransferase.
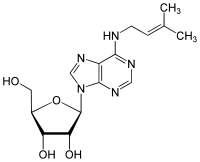 N6-Isopentenyladenosine
N6-Isopentenyladenosine
Structural studies
As of late 2007, only one structure has been solved for this class of enzymes, with the PDB accession code 2QGN.
References
Literature
- Kline LK, Fittler F, Hall RH (1969). "N6-(Δ2-isopentenyl)adenosine. Biosynthesis in transfer ribonucleic acid in vitro". Biochemistry. 8 (11): 4361–4371. doi:10.1021/bi00839a021. PMID 4311031.
- Rosenbaum N, Gefter ML (1972). "Δ2-Isopentenylpyrophosphate: Transfer Ribonucleic Acid Δ2-Isopentenyltransferase from Escherichia coli. Purification and properties of the enzyme". J. Biol. Chem. 247 (18): 5675–5680. PMID 4341485.