Scandium perchlorate
Scandium perchlorate is an inorganic compound with the chemical formula Sc(ClO4)3.[3][4][5]
 | |
| Identifiers | |
|---|---|
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.034.453 |
| EC Number |
|
PubChem CID |
|
| UN number | 3098 |
| Properties | |
| Sc(ClO4)3 | |
| Molar mass | 343.308[2] |
| Density | 1.449[1] |
| Solubility | soluble[1] |
| Vapor pressure | 0.12[1] |
| Hazards | |
| GHS pictograms | 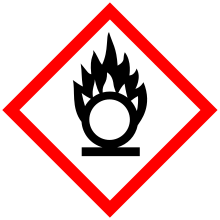  |
| GHS Signal word | Danger |
GHS hazard statements |
H272, H314 |
| P210, P220, P221, P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P370+378, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
Scandium perchlorate can be prepared by dissolving scandium oxide in perchloric acid:
gollark: --search bees
gollark: --search bee
gollark: Oops.
gollark: --search !wen bee
gollark: --magic reload_ext duckduckgo
References
- "高氯酸钪 | 14066-05-8". Chembook. August 2017.
- "Scandium triperchlorate | Cl3O12Sc | ChemSpider". ChemSpider. 2017-08-29.
- 袁景利; 谭民裕 (1987). "稀土与芳香胺及其氮氧化物形成配合物的研究(Ⅵ)——钪、钇高氯酸盐与2,2'-联喹啉-N,N'-二氧化物固体配合物的合成及性质". 《稀土》 , 1987 (1) :7-11 (1): 7-11. Retrieved 2017-08-29.
- 何良友; 甘新民; 谭民裕 (1990). "钪和钇高氯酸盐与2,2'-二喹啉甲烷固体配合物的合成及性质". 《成都科技大学学报》 (4): 129-133. Retrieved 2017-08-29.
- 甘新民; 谭民裕 (1988). "高氯酸钪、钇与1,8-萘啶氮氧化物固体配合物的合成及性质". 《高等学校化学学报》 (3): 78-80. Retrieved 2017-08-29.
- 刘伟生; 谭民俗 (1990). "1,10-二氮杂菲-1-氧化物与高氯酸钪、钇的配合物". 《化学研究与应用》 (3): 99-101. Retrieved 2017-08-29.
- 甘新民; 唐宁; 张文军; 谭民裕 (1989). "1,8-萘啶氮氧化物钪(Ⅲ)配合物的合成与性质". 《无机化学学报》. 5 (3): 17-21. Retrieved 2017-08-29.CS1 maint: multiple names: authors list (link)
Compounds containing perchlorate group
| HClO4 | He | ||||||||||||||||
| LiClO4 | Be(ClO4)2 | B(ClO 4)− 4 B(ClO4)3 |
ROClO3 | N(ClO4)3 NH4ClO4 NOClO4 |
O | FClO4 | Ne | ||||||||||
| NaClO4 | Mg(ClO4)2 | Al(ClO4)3 | Si | P | S | ClO− 4 ClOClO3 Cl2O7 |
Ar | ||||||||||
| KClO4 | Ca(ClO4)2 | Sc(ClO4)3 | Ti(ClO4)4 | VO(ClO4)3 VO2(ClO4) |
Cr(ClO4)3 | Mn(ClO4)2 | Fe(ClO4)3 | Co(ClO4)2, Co(ClO4)3 |
Ni(ClO4)2 | Cu(ClO4)2 | Zn(ClO4)2 | Ga(ClO4)3 | Ge | As | Se | Br | Kr |
| RbClO4 | Sr(ClO4)2 | Y(ClO4)3 | Zr(ClO4)4 | Nb(ClO4)5 | Mo | Tc | Ru | Rh(ClO4)3 | Pd(ClO4)2 | AgClO4 | Cd(ClO4)2 | In(ClO4)3 | Sn(ClO4)4 | Sb | TeO(ClO4)2 | I | Xe |
| CsClO4 | Ba(ClO4)2 | Hf(ClO4)4 | Ta(ClO4)5 | W | Re | Os | Ir | Pt | Au | Hg2(ClO4)2, Hg(ClO4)2 |
Tl(ClO4), Tl(ClO4)3 |
Pb(ClO4)2 | Bi(ClO4)3 | Po | At | Rn | |
| FrClO4 | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce(ClO4)x | Pr | Nd | Pm | Sm(ClO4)3 | Eu(ClO4)3 | Gd(ClO4)3 | Tb(ClO4)3 | Dy(ClO4)3 | Ho(ClO4)3 | Er(ClO4)3 | Tm(ClO4)3 | Yb(ClO4)3 | Lu(ClO4)3 | |||
| Ac | Th(ClO4)4 | Pa | UO2(ClO4)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.