Copper(II) perchlorate
Copper(II) perchlorate is a salt of copper and perchloric acid. It is a hygroscopic crystalline blue solid, most commonly copper(II) perchlorate hexahydrate, Cu(ClO4)2·6H2O. Like any perchlorate, it is a strong oxidizing agent.
_perchlorate_structure.png) | |
| Names | |
|---|---|
| IUPAC name
Copper(II) perchlorate | |
| Other names
Cupric perchlorate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.978 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Cu(ClO4)2 | |
| Molar mass | 262.447 g/mol (anhydrous) 370.539 g/mol (hexahydrate) |
| Appearance | Blue solid hygroscopic[1] |
| Odor | odorless |
| Density | 2.225 g/cm3 |
| Melting point | 82 °C (180 °F; 355 K) |
| Boiling point | 120 °C (248 °F; 393 K) |
| 146 g/100ml (30°C) | |
Refractive index (nD) |
1.505[2] |
| Hazards | |
| Safety data sheet | External MSDS |
| GHS pictograms | 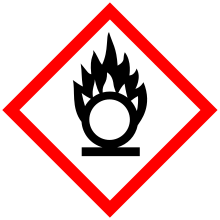  |
| GHS Signal word | Warning |
GHS hazard statements |
H272, H315, H319, H335 |
| P210, P220, P221, P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P403+233, P405, P501 | |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 1 mg/m3 (as Cu)[3] |
REL (Recommended) |
TWA 1 mg/m3 (as Cu)[3] |
IDLH (Immediate danger) |
TWA 100 mg/m3 (as Cu)[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- http://www.chemspider.com/Chemical-Structure.26246.html
- http://www.chemicalbook.com/ChemicalProductProperty_EN_CB2471598.htm
- NIOSH Pocket Guide to Chemical Hazards. "#0150". National Institute for Occupational Safety and Health (NIOSH).
Compounds containing perchlorate group
| HClO4 | He | ||||||||||||||||
| LiClO4 | Be(ClO4)2 | B(ClO 4)− 4 B(ClO4)3 |
ROClO3 | N(ClO4)3 NH4ClO4 NOClO4 |
O | FClO4 | Ne | ||||||||||
| NaClO4 | Mg(ClO4)2 | Al(ClO4)3 | Si | P | S | ClO− 4 ClOClO3 Cl2O7 |
Ar | ||||||||||
| KClO4 | Ca(ClO4)2 | Sc(ClO4)3 | Ti(ClO4)4 | VO(ClO4)3 VO2(ClO4) |
Cr(ClO4)3 | Mn(ClO4)2 | Fe(ClO4)3 | Co(ClO4)2, Co(ClO4)3 |
Ni(ClO4)2 | Cu(ClO4)2 | Zn(ClO4)2 | Ga(ClO4)3 | Ge | As | Se | Br | Kr |
| RbClO4 | Sr(ClO4)2 | Y(ClO4)3 | Zr(ClO4)4 | Nb(ClO4)5 | Mo | Tc | Ru | Rh(ClO4)3 | Pd(ClO4)2 | AgClO4 | Cd(ClO4)2 | In(ClO4)3 | Sn(ClO4)4 | Sb | TeO(ClO4)2 | I | Xe |
| CsClO4 | Ba(ClO4)2 | Hf(ClO4)4 | Ta(ClO4)5 | W | Re | Os | Ir | Pt | Au | Hg2(ClO4)2, Hg(ClO4)2 |
Tl(ClO4), Tl(ClO4)3 |
Pb(ClO4)2 | Bi(ClO4)3 | Po | At | Rn | |
| FrClO4 | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce(ClO4)x | Pr | Nd | Pm | Sm(ClO4)3 | Eu(ClO4)3 | Gd(ClO4)3 | Tb(ClO4)3 | Dy(ClO4)3 | Ho(ClO4)3 | Er(ClO4)3 | Tm(ClO4)3 | Yb(ClO4)3 | Lu(ClO4)3 | |||
| Ac | Th(ClO4)4 | Pa | UO2(ClO4)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.