Hydroxy alpha sanshool
Hydroxy-alpha sanshool is a molecule found in plants from the genus Zanthoxylum. It is believed to be responsible for the numbing and tingling sensation caused by eating food cooked with Sichuan peppercorns.
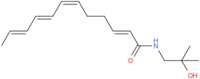 | |
| Names | |
|---|---|
| IUPAC name
(2E,6Z,8E,10E)-N-(2-hydroxy-2-methylpropyl)dodeca-2,6,8,10-tetraenamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C16H25NO2 | |
| Molar mass | 263.381 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The term sanshool in the compound's name is derived from the Japanese term for the Sichuan pepper, sanshō (山椒) (literally, mountain pepper), to which was appended the suffix -ol, indicating an alcohol.
Mechanism
The chemical structure of hydroxy-alpha sanshool is similar to that of capsaicin, but the mechanism of action by which it induces nerve sensations have been a matter of debate. Although the compound is an agonist at the pain-integration channels TRPV1 and TRPA1 as capsaicin, newer evidence suggests that the tandem pore domain potassium channels KCNK3, KCNK9, and KCNK18 are primarily responsible for sanshool's effects.[1]
Hydroxy-alpha sanshool excites D-hair afferent nerve fibers, a distinct subset of the sensitive light touch receptors in the skin, and targets novel populations of Aβ and C-fiber nerve fibers.[2]
Extraction
To isolate the molecule from the pepper in form of an extract, steam distillation can be used: Dried peels of the fruit are immersed in a mixture of lower alcohols (for example ethanol) and water with a mass percentage between 35-65% of the alcohol. The solution gets heated up in the process of steam distillation where the aqueous part evaporates and takes parts of the hydroxy-alpha- sanshool up, too. The distillate separates in two phases: the aqueous ethanol phase and the oil phase which contains the desired molecule.
A problem with this extraction method is the low yield which is most times below 60%.[3]
See also
References
- Bautista DM, Sigal YM, Milstein AD, Garrison JL, Zorn JA, Tsuruda PR, Nicoll RA, Julius D (July 2008). "Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels". Nat. Neurosci. 11 (7): 772–9. doi:10.1038/nn.2143. PMC 3072296. PMID 18568022.
- Lennertz, Richard C; Tsunozaki, Makoto; Bautista, Diana M; Stucky, Cheryl L (24 Mar 2010). "Physiological basis of tingling paresthesia evoked by hydroxy-α-sanshool". J. Neurosci. Society for Neuroscience. 30 (12): 4353–4361. doi:10.1523/JNEUROSCI.4666-09.2010. PMC 2852189. PMID 20335471.
- Schlander, David; Schleif, Chiara; Schnell, Maxim; Roumeliotis, Paul. "Hydroxy-alpha-sanshool" (PDF). Technische Universität Darmstadt: 2. Cite journal requires
|journal=(help)