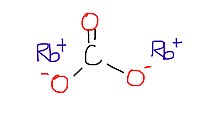
Rubidium carbonate
Rubidium carbonate, Rb2CO3, is a convenient compound of rubidium; it is stable, not particularly reactive, and readily soluble in water, and is the form in which rubidium is usually sold.
 | |
| Names | |
|---|---|
| IUPAC name
Rubidium carbonate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.008.666 |
PubChem CID |
|
| RTECS number |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Rb2CO3 | |
| Molar mass | 230.945 g/mol |
| Appearance | White powder, very hygroscopic |
| Melting point | 837 °C (1,539 °F; 1,110 K)[1] |
| Boiling point | 900 °C (1,650 °F; 1,170 K) (decomposes) |
| Very soluble | |
| −75.4·10−6 cm3/mol | |
| Hazards | |
| Main hazards | Irritant |
| Flash point | Non-flammable |
| Related compounds | |
Other cations |
Lithium carbonate Sodium carbonate Potassium carbonate Caesium carbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
It can be prepared by adding ammonium carbonate to rubidium hydroxide.[2]
Uses
It is used in some kinds of glass-making by enhancing stability and durability as well as reducing its conductivity. It is also used as a part of a catalyst for preparing short-chain alcohols from feed gas.[3]
gollark: I'm probably just going to be eternally stuck with advancing and somewhat bad modern computers plus random fiddling with other ones.
gollark: Which are still general purpose computers.
gollark: You aren't going to produce your own usably sized FPGA *or* recent CPU so it's probably most sensible to just go for really common and more practical devices.
gollark: FPGAs remain quite costly and niche.
gollark: Much more readily available, very multipurpose, still pretty fast.
References
- Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 23 (11th ed.). Cambridge University Press. p. 809.
- Canada Patents
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.