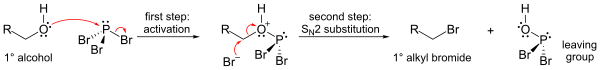Phosphorus tribromide
Phosphorus tribromide is a colourless liquid with the formula PBr3. The liquid fumes in moist air due to hydrolysis and has a penetrating odour. It is used in the laboratory for the conversion of alcohols to alkyl bromides.
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Phosphorus tribromide | |
| Other names
phosphorus(III) bromide, phosphorous bromide, tribromophosphine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.253 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| PBr3 | |
| Molar mass | 270.69 g/mol |
| Appearance | clear, colourless liquid |
| Density | 2.852 g/cm3 |
| Melting point | −41.5 °C (−42.7 °F; 231.7 K) |
| Boiling point | 173.2 °C (343.8 °F; 446.3 K) |
| rapid hydrolysis | |
Refractive index (nD) |
1.697 |
| Viscosity | 0.001302 Pas |
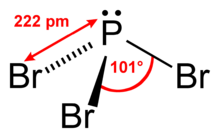
| Structure | |
| trigonal pyramidal | |
| Hazards | |
| Safety data sheet | See: data page |
EU classification (DSD) (outdated) |
|
| R-phrases (outdated) | R14, R34, R37 |
| S-phrases (outdated) | (S1/2), S26, S45 |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions |
phosphorus trifluoride phosphorus trichloride phosphorus triiodide |
Other cations |
nitrogen tribromide arsenic tribromide antimony tribromide |
Related compounds |
phosphorus pentabromide phosphorus oxybromide |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
PBr3 is prepared by treating red phosphorus with bromine. An excess of phosphorus is used in order to prevent formation of PBr5:[1][2]
- 2 P + 3 Br2 → 2 PBr3
Because the reaction is highly exothermic, it is often conducted in the presence of a diluent such as PBr3.
Reactions
Phosphorus tribromide, like PCl3 and PF3, has both properties of a Lewis base and a Lewis acid. For example, with a Lewis acid such as boron tribromide it forms stable 1 :1 adducts such as Br3B · PBr3. At the same time PBr3 can react as an electrophile or Lewis acid in many of its reactions, for example with amines.
The most important reaction of PBr3 is with alcohols, where it replaces an OH group with a bromine atom to produce an alkyl bromide. All three bromides can be transferred.
- PBr3 + 3 ROH → 3 RBr + HP(O)(OH)2
The mechanism (shown for a primary alcohol) involves formation of a phosphorus ester (to form a good leaving group), followed by an SN2 substitution.
Because of the SN2 substitution step, the reaction generally works well for primary and secondary alcohols, but fails for tertiary alcohols. If the reacting carbon centre is chiral, the reaction usually occurs with inversion of configuration at the alcohol alpha carbon, as is usual with an SN2 reaction.
In a similar reaction, PBr3 also converts carboxylic acids to acyl bromides.
- PBr3 + 3 RCOOH → 3 RCOBr + HP(O)(OH)2
Applications
The main use for phosphorus tribromide is for conversion of primary or secondary alcohols to alkyl bromides,[3] as described above. PBr3 usually gives higher yields than hydrobromic acid, and it avoids problems of carbocation rearrangement- for example even neopentyl bromide can be made from the alcohol in 60% yield.[4]
Another use for PBr3 is as a catalyst for the α-bromination of carboxylic acids. Although acyl bromides are rarely made in comparison with acyl chlorides, they are used as intermediates in Hell-Volhard-Zelinsky halogenation.[5] Initially PBr3 reacts with the carboxylic acid to form the acyl bromide, which is more reactive towards bromination. The overall process can be represented as
On a commercial scale, phosphorus tribromide is used in the manufacture of pharmaceuticals such as alprazolam, methohexital and fenoprofen. It is also a potent fire suppression agent marketed under the name PhostrEx.
Precautions
PBr3 evolves corrosive HBr, is toxic, and reacts violently with water and alcohols.
- PBr3 + 3 H2O → H3PO3 + 3 HBr
In reactions that produce phosphorous acid as a by-product, when working up by distillation be aware that this can decompose above about 160 °C to give phosphine which can cause explosions in contact with air.[3]
References
- J. F. Gay, R. N. Maxson "Phosphorus(III) Bromide" Inorganic Syntheses, 1947, vol. 2, 147ff. doi:10.1002/9780470132333.ch43
- Burton, T. M.; Degerping, E. F. (1940). "The Preparation of Acetyl Bromide". Journal of the American Chemical Society. 62 (1): 227. doi:10.1021/ja01858a502.
- Harrison, G. C.; Diehl, H. (1955). "β-Ethoxyethyl Bromide". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 3, p. 370
- Wade, L. G. Jr. (2005). Organic Chemistry (6th ed.). Upper Saddle River, NJ, USA: Pearson/Prentice Hall. p. 477.
- Wade, L. G. Jr. (2005). Organic Chemistry (6th ed.). Upper Saddle River, NJ, USA: Pearson/Prentice Hall. p. 1051.
Further reading
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Lide, D. R., ed. (1990). Handbook of Chemistry and Physics (71st ed.). Ann Arbor, MI: CRC Press. ISBN 978-0849304712.
- March, J. (1992). Advanced Organic Chemistry (4th ed.). New York: Wiley. p. 723. ISBN 978-0471601807.
- Stecher, P. G., ed. (1960). The Merck Index (7th ed.). Rahway, NJ, USA: Merck & Co.
- Holmes, R. R. (1960). "An Examination of the Basic Nature of the Trihalides of Phosphorus, Arsenic and Antimony". Journal of Inorganic and Nuclear Chemistry. 12 (3–4): 266–275. doi:10.1016/0022-1902(60)80372-7.