Mercury(II) bromide
Mercury(II) bromide or mercuric bromide is the chemical compound composed of mercury and bromine with the formula HgBr2. This white crystalline solid is a laboratory reagent. Like mercury(II) chloride, it is extremely toxic.
 | |
| Names | |
|---|---|
| IUPAC name
Mercury(II) bromide | |
| Other names
Mercuric bromide | |
| Identifiers | |
| ECHA InfoCard | 100.029.245 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| Properties | |
| HgBr2 | |
| Molar mass | 360.41 g/mol |
| Appearance | white solid |
| Density | 6.03 g/cm3, solid |
| Melting point | 237 °C (459 °F; 510 K) |
| Boiling point | 322 °C (612 °F; 595 K) |
| soluble | |
| Solubility | very slightly soluble in ether |
| −94.2·10−6 cm3/mol | |
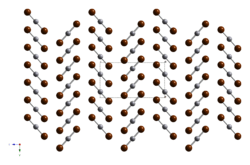
| Structure | |
| rhombic | |
| Hazards | |
EU classification (DSD) (outdated) |
|
| R-phrases (outdated) | R26/27/28, R33, R50/53 |
| S-phrases (outdated) | (S1/2), S13, S28, S45, S60, S61 |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions |
Mercury(II) fluoride Mercury(II) chloride Mercury(II) iodide |
Other cations |
Zinc bromide Cadmium bromide Mercury(I) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Mercury(II) bromide can be manufactured by: adding potassium bromide to a solution of mercuric salt and crystallizing; by precipitation using a mercury(II) nitrate and sodium bromide solution; by dissolving mercury(II) oxide in hydrobromic acid. Also, Mercury(II) bromide can be created by reacting mercury with bromine.
Reactions
Mercury(II) bromide is used as a reagent in the Koenigs–Knorr reaction, which forms glycoside linkages on carbohydrates.[1][2]
It is also used to test for the presence of arsenic, as recommended by the Pharmacopoeia.[3] The arsenic in the sample is first converted to arsine gas by treatment with hydrogen. Arsine reacts with mercury(II) bromide:[4]
The white mercury(II) bromide will turn yellow, brown, or black if arsenic is present in the sample.[5]
Mercury(II) bromide reacts violently with elemental indium at high temperatures[6] and, when exposed to potassium, can form shock-sensitive explosive mixtures.[7]
References
- Horton, Derek (2004), Advances in Carbohydrate Chemistry and Biochemistry, Amsterdam: Elseveir Academic Press, p. 76, ISBN 0-12-007259-9, retrieved 2008-05-29
- Stick, Robert V. (2001), Carbohydrates: The Sweet Molecules of Life, San Diego: Academic Press, p. 125, ISBN 0-12-670960-2, retrieved 2008-05-29
- Pederson, Ole (2006), Pharmaceutical Chemical Analysis, Boca Raton, Florida: CRC Press, p. 107, ISBN 0-8493-1978-1, retrieved 2008-05-29
- Odegaard, Nancy; Sadongei, Alyce (2005), Old Poisons, New Problems, Rowman Altamira, p. 58, ISBN 0-7591-0515-4, retrieved 2008-05-29
- Townsend, Timothy G.; Solo-Gabriele, Helena (2006), Environmental Impacts of Treated Wood, Boca Raton, Florida: CRC Press, p. 339, ISBN 0-8493-6495-7, retrieved 2008-05-29
- Bretherick, L.; Urben, P. G.; Pitt, Martin John (1999), Bretherick's Handbook of Reactive Chemical Hazards, Elseveir Academic Press, p. 110, ISBN 0-7506-3605-X
- Bretherick, L.; Urben, P. G.; Pitt, Martin John (1999), Bretherick's Handbook of Reactive Chemical Hazards, Elseveir Academic Press, p. 1276, ISBN 0-7506-3605-X
