Urokinase
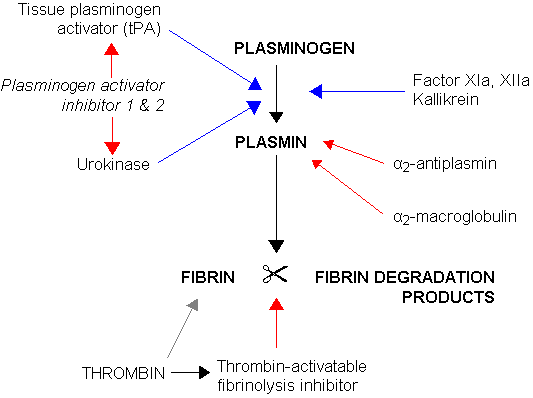
Urokinase, also known as urokinase-type plasminogen activator (uPA), is a serine protease present in humans and other animals. The human urokinase protein was discovered, but not named, by McFarlane and Pilling in 1947.[5] Urokinase was originally isolated from human urine, and it is also present in the blood and in the extracellular matrix of many tissues. The primary physiological substrate of this enzyme is plasminogen, which is an inactive form (zymogen) of the serine protease plasmin. Activation of plasmin triggers a proteolytic cascade that, depending on the physiological environment, participates in thrombolysis or extracellular matrix degradation. This cascade had been involved in vascular diseases and cancer progression.[6]
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| ATC code | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C1376H2145N383O406S18 |
| Molar mass | 31126.65 g·mol−1 |
| | |
Urokinase is encoded in humans by the PLAU gene, which stands for "plasminogen activator, urokinase".[7] The same symbol represents the gene in other animal species.
Function
The PLAU gene encodes a serine protease (EC 3.4.21.73) involved in degradation of the extracellular matrix and possibly tumor cell migration and proliferation. A specific polymorphism in this gene may be associated with late-onset Alzheimer disease and also with decreased affinity for fibrin-binding. The protein encoded by this gene converts plasminogen to plasmin by specific cleavage of an Arg-Val bond in plasminogen. This gene's proprotein is cleaved at a Lys-Ile bond by plasmin to form a two-chain derivative in which a single disulfide bond connects the amino-terminal A-chain to the catalytically active, carboxy-terminal B-chain. This two-chain derivative is also called HMW-uPA (high molecular weight uPA). HMW-uPA can be further processed into LMW-uPA (low molecular weight uPA) by cleavage of chain A into a short chain A (A1) and an amino-terminal fragment. LMW-uPA is proteolytically active but does not bind to the uPA receptor.[8]
Structure
Urokinase is a 411-residue protein, consisting of three domains: the serine protease domain, the kringle domain, and the growth factor domain. Urokinase is synthesized as a zymogen form (prourokinase or single-chain urokinase), and is activated by proteolytic cleavage between Lys158 and Ile159. The two resulting chains are kept together by a disulfide bond.
Interaction partners
The most important inhibitors of urokinase are the serpins plasminogen activator inhibitor-1 (PAI-1) and plasminogen activator inhibitor-2 (PAI-2), which inhibit the protease activity irreversibly. In the extracellular matrix, urokinase is tethered to the cell membrane by its interaction to the urokinase receptor.
uPa also interacts with protein C inhibitor.[9][10]
Urokinase and cancer
Elevated expression levels of urokinase and several other components of the plasminogen activation system are found to be correlated with tumor malignancy. It is believed that the tissue degradation following plasminogen activation facilitates tissue invasion and, thus, contributes to metastasis.[11] Urokinase-type plasminogen activator (uPA) is more commonly associated with cancer progression than tissue plasminogen activator (tPA).[12] This makes uPA an attractive drug target, and, so, inhibitors have been sought to be used as anticancer agents.[13][14] However, incompatibilities between the human and murine systems hamper clinical evaluation of these agents. Moreover, urokinase is used by normal cells for tissue remodeling and vessel growth, which necessitates distinguishing cancer-associated urokinase features for specific targeting.[11]
uPA breakdown of the extracellular matrix is crucial for initiating the angiogenesis which is associated with cancer growth.[12]
uPA antigen is elevated in breast cancer tissue, which correlates with poor prognosis in breast cancer patients.[12] For this reason, uPA can be used as a diagnostic biomarker in breast cancer.[12]
Through its interaction with the urokinase receptor, urokinase affects several other aspects of cancer biology such as cell adhesion, migration, and cellular mitotic pathways.
As of December 7, 2012, Mesupron (upamostat), a small molecule serine protease inhibitor developed by the WILEX pharmaceutical company, has completed phase II trials.[15] Mesupron appears to be safe when combined with chemotherapeutic drug Capecitabine for the progression-free survival in human breast cancer.[16]
Clinical applications
Urokinase is effective for the restoration of flow to intravenous catheters blocked by clotted blood or fibrin (catheter clearance). Catheters are used extensively to administer treatments to patients for such purposes as dialysis, nutrition, antibiotic treatment and cancer treatment. Approximately 25% of catheters become blocked, meaning that affected patients cannot receive treatment until the catheter has been cleared or replaced. Urokinase is also used clinically as a thrombolytic agent in the treatment of severe or massive deep venous thrombosis, peripheral arterial occlusive disease, pulmonary embolism, acute myocardial infarction (AMI, heart attack), and occluded dialysis cannulas (catheter clearance). It is also administered intrapleurally to improve the drainage of complicated pleural effusions and empyemas. Urokinase is marketed as Kinlytic (formerly Abbokinase) and competes with recombinant tissue plasminogen activator (e.g., alteplase) as a thrombolytic drug.
All plasminogen activators (urokinase, tPA) catalyze the production of plasmin, which in turn leads to the breakdown of the fibrin lattice structure in blood clots. While there are commonalities in the mode of action for urokinase and tPA, urokinase has some advantages for treatment of peripheral clots (Pulmonary Embolism, Deep Vein Thrombosis, Peripheral arterial occlusive disease).
Unlike tPA, which is activated by binding to the fibrin within clots, urokinase is not sequestered by fibrin and therefore does not specifically attack hemostatic clots. This makes urokinase less likely to break down such hemostatic clots that are essential for ongoing blood vessel repair throughout the body. Dissolution of these “good” clots can lead to serious adverse events through hemorrhagic bleeding. Years of clinical study have confirmed the safety advantage of using urokinase.[17][18] Consequently, urokinase has been preferentially used in deep venous thrombosis and peripheral arterial occlusive disease where it is administered directly to the site of the clot while tPA is preferred in AMI where peripheral bleeding is a secondary consideration.
Society and culture
The presence of a fibrinolytic enzyme in human urine was reported in 1947, without a name given for such an enzyme behind its effect.[19] In 1952 a purified form of the enzyme was extracted from human urine and named "urokinase" for "urinary kinase".[20] The full text for this article is lost, and the only citation points to the abstract of a list of papers read at a conference in the same journal.[21] A few other papers on the purification were published independently around the same time. By 1960, it was still unclear whether the activation of plasminogen has anything to do with a protease, but a kinase is thought to play a role regardless.[22]
References
- GRCh38: Ensembl release 89: ENSG00000122861 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000021822 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Degryse B (1 June 2011). "The urokinase receptor system as strategic therapeutic target: challenges for the 21st century". Current Pharmaceutical Design. 17 (19): 1872–3. doi:10.2174/138161211796718161. PMID 21711231.
- Tang L, Han X (March 2013). "The urokinase plasminogen activator system in breast cancer invasion and metastasis". Biomedicine & Pharmacotherapy. 67 (2): 179–82. doi:10.1016/j.biopha.2012.10.003. PMID 23201006.
- Nagai M, Hiramatsu R, Kanéda T, Hayasuke N, Arimura H, Nishida M, Suyama T (Dec 1985). "Molecular cloning of cDNA coding for human preprourokinase". Gene. 36 (1–2): 183–8. doi:10.1016/0378-1119(85)90084-8. PMID 2415429.
- "Entrez Gene: PLAU plasminogen activator, urokinase".
- Geiger M, Huber K, Wojta J, Stingl L, Espana F, Griffin JH, Binder BR (August 1989). "Complex formation between urokinase and plasma protein C inhibitor in vitro and in vivo". Blood. 74 (2): 722–8. doi:10.1182/blood.V74.2.722.722. PMID 2752144.
- España F, Berrettini M, Griffin JH (August 1989). "Purification and characterization of plasma protein C inhibitor". Thrombosis Research. 55 (3): 369–84. doi:10.1016/0049-3848(89)90069-8. PMID 2551064.
- Josip Madunić (2018). "The Urokinase Plasminogen Activator System in Human Cancers: An Overview of Its Prognostic and Predictive Role". Thrombosis and Haemostasis. 118 (12): 2020–2036. doi:10.1055/s-0038-1675399. PMID 30419600.
- Mahmood N, Mihalcioiu C, Rabbani SA (2018). "T Cells Engineered to Target Senescence". Frontiers in Oncology. 8: 24. doi:10.3389/fonc.2018.00024. PMC 5816037. PMID 29484286.
- Jankun J, Skrzypczak-Jankun E (July 1999). "Molecular basis of specific inhibition of urokinase plasminogen activator by amiloride". Cancer Biochemistry Biophysics. 17 (1–2): 109–23. PMID 10738907.
- Matthews H, Ranson M, Kelso MJ (November 2011). "Anti-tumour/metastasis effects of the potassium-sparing diuretic amiloride: an orally active anti-cancer drug waiting for its call-of-duty?". International Journal of Cancer. 129 (9): 2051–61. doi:10.1002/ijc.26156. PMID 21544803.
- "Gemcitabine With or Without WX-671 in Treating Patients With Locally Advanced Pancreatic Cancer That Cannot Be Removed By Surgery". ClinicalTrials.gov.
- "Fox Chase Cancer Center : New Small Molecule Inhibitor Could be a Safe and First-Line Treatment for Metastatic Breast Cancer". Press Release. Temple University Health System.
- Ouriel, K.; et al. (2000). "Complications Associated with the Use of Urokinase and Recombinant Tissue Plasminogen Activator for Catheter-directed Peripheral Arterial and Venous Thrombolysis". JVIR. 11 (3): 295–298. doi:10.1016/S1051-0443(07)61420-1. PMID 10735422.
- Cina, C.; et al. (1999). "Intraarterial Catheter-Directed Thrombolysis: Urokinase versus Tissue Plasminogen Activator". Ann Vasc Surg. 13 (6): 571–575. doi:10.1007/s100169900300. PMID 10541608. S2CID 470599.
- Macfarlane RG, Pilling J (June 1947). "Fibrinolytic activity of normal urine". Nature. 159 (4049): 779. Bibcode:1947Natur.159Q.779M. doi:10.1038/159779a0. PMID 20241608. S2CID 4125748.
- Sobel GW, Mohler SR, Jones NW, Dowdy ABC, Guest MM. Urokinase: an activator of plasma profibrinolysin extracted from urine. Am J Physiol 1952; 171: 768-69.
- "Abstracts of Papers Read". American Journal of Physiology. Legacy Content. 171 (3): 704–781. 30 November 1952. doi:10.1152/ajplegacy.1952.171.3.704.
Normal human and dog urine contains fibrinolysin (plasmin) and a potent activator of profibrinolysin (plasminogen). The activator, which we have designated urokinase, can be concentrated and partially purified by acetone or alcohol fractionation methods.
- Celander DR, Guest MM (August 1960). "The biochemistry and physiology of urokinase". The American Journal of Cardiology. 6 (2): 409–19. doi:10.1016/0002-9149(60)90333-7. PMID 13808740.
Further reading
- Ploug M, Gårdsvoll H, Jørgensen TJ, Lønborg Hansen L, Danø K (April 2002). "Structural analysis of the interaction between urokinase-type plasminogen activator and its receptor: a potential target for anti-invasive cancer therapy". Biochemical Society Transactions. 30 (2): 177–83. doi:10.1042/BST0300177. PMID 12023847.
- Alfano M, Sidenius N, Blasi F, Poli G (November 2003). "The role of urokinase-type plasminogen activator (uPA)/uPA receptor in HIV-1 infection". Journal of Leukocyte Biology. 74 (5): 750–6. doi:10.1189/jlb.0403176. PMID 12960238.
- Harbeck N, Kates RE, Gauger K, Willems A, Kiechle M, Magdolen V, Schmitt M (March 2004). "Urokinase-type plasminogen activator (uPA) and its inhibitor PAI-I: novel tumor-derived factors with a high prognostic and predictive impact in breast cancer". Thrombosis and Haemostasis. 91 (3): 450–6. doi:10.1160/TH03-12-0798. PMID 14983219. S2CID 19904733.
- Gilabert-Estelles J, Ramon LA, España F, Gilabert J, Castello R, Estelles A (2006). "Expression of the fibrinolytic components in endometriosis". Pathophysiology of Haemostasis and Thrombosis. 35 (1–2): 136–40. doi:10.1159/000093556. PMID 16855359. S2CID 29270171.