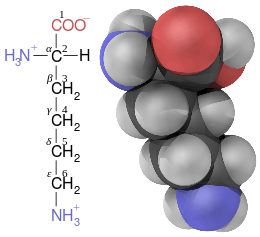
Non-proteinogenic amino acids
In biochemistry, non-coded or non-proteinogenic amino acids are those not naturally encoded or found in the genetic code of any organism. Despite the use of only 22 amino acids (21 in eukaryotes[note 1]) by the translational machinery to assemble proteins (the proteinogenic amino acids), over 140 amino acids are known to occur naturally in proteins and thousands more may occur in nature or be synthesized in the laboratory.[1] Many non-proteinogenic amino acids are noteworthy because they are;
- intermediates in biosynthesis,
- post-translationally formed in proteins,
- possess a physiological role (e.g. components of bacterial cell walls, neurotransmitters and toxins),
- natural or man-made pharmacological compounds,
- present in meteorites and in prebiotic experiments (e.g. Miller–Urey experiment).
Definition by negation
Technically, any organic compound with an amine (-NH2) and a carboxylic acid (-COOH) functional group is an amino acid. The proteinogenic amino acids are small subset of this group that possess central carbon atom (α- or 2-) bearing an amino group, a carboxyl group, a side chain and an α-hydrogen levo conformation, with the exception of glycine, which is achiral, and proline, whose amine group is a secondary amine and is consequently frequently referred to as an imino acid for traditional reasons, albeit not an imino.
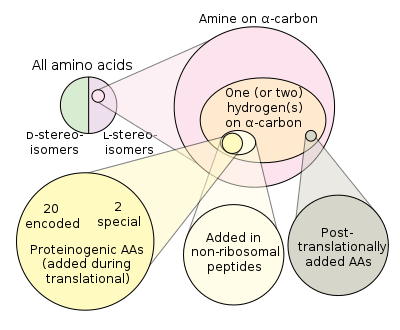
The genetic code encodes 20 standard amino acids for incorporation into proteins during translation. However, there are two extra proteinogenic amino acids: selenocysteine and pyrrolysine. These non-standard amino acids do not have a dedicated codon, but are added in place of a stop codon when a specific sequence is present, UGA codon and SECIS element for selenocysteine,[2] UAG PYLIS downstream sequence for pyrrolysine.[3] All other amino acids are termed "non-proteinogenic".
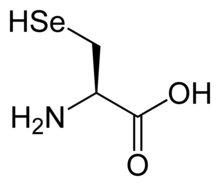 Selenocysteine. This amino acid contains a selenol group on its β-carbon
Selenocysteine. This amino acid contains a selenol group on its β-carbon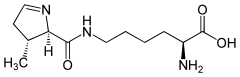 Pyrrolysine. This amino acid is formed by joining to the ε-amino group of lysine a carboxylated pyrroline ring
Pyrrolysine. This amino acid is formed by joining to the ε-amino group of lysine a carboxylated pyrroline ring
There are various groups of amino acids:[4]
- 20 standard amino acids
- 22 proteinogenic amino acids
- over 80 amino acids created abiotically in high concentrations
- about 900 are produced by natural pathways
- over 118 engineered amino acids have been placed into protein
These groups overlap, but are not identical. All 22 proteinogenic amino acids are biosynthesised by organisms and some, but not all, of them also are abiotic (found in prebiotic experiments and meteorites). Some natural amino acids, such as norleucine, are misincorporated translationally into proteins due to infidelity of the protein-synthesis process. Many amino acids, such as ornithine, are metabolic intermediates produced biosynthetically, but not incorporated translationally into proteins. Post-translational modification of amino-acid residues in proteins leads to the formation of many proteinaceous, but non-proteinogenic, amino acids. Other amino acids are solely found in abiotic mixes (e.g. α-methylnorvaline). Over 30 unnatural amino acids have been inserted translationally into protein in engineered systems, yet are not biosynthetic.[4]
Nomenclature
In addition to the IUPAC numbering system to differentiate the various carbons in an organic molecule, by sequentially assigning a number to each carbon, including those forming a carboxylic group, the carbons along the side-chain of amino acids can also be labelled with Greek letters, where the α-carbon is the central chiral carbon possessing a carboxyl group, a side chain and, in α-amino acids, an amino group – the carbon in carboxylic groups is not counted.[5] (Consequently, the IUPAC names of many non-proteinogenic α-amino acids start with 2-amino- and end in -ic acid.)
Natural, but non L-α-amino acids
Most natural amino acids are α-amino acids in the L conformation, but some exceptions exist.
Non-alpha
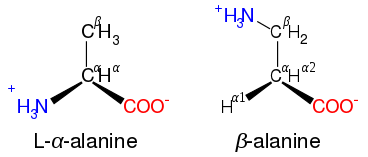
Some non-α amino acids exist in organisms. In these structures, the amine group displaced further from the carboxylic acid end of the amino acid molecule. Thus a β amino acid has the amine group bonded to the second carbon away, and a γ amino acid has it on the third. Examples include β-alanine, GABA, and δ-aminolevulinic acid.
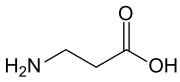 β-alanine: an amino acid produced by aspartate 1-decarboxylase and a precursor to coenzyme A[6] and the peptides carnosine and anserine.
β-alanine: an amino acid produced by aspartate 1-decarboxylase and a precursor to coenzyme A[6] and the peptides carnosine and anserine. γ-Aminobutyric acid (GABA): a neurotransmitter in animals.
γ-Aminobutyric acid (GABA): a neurotransmitter in animals.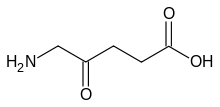 δ-Aminolevulinic acid: an intermediate in tetrapyrrole biosynthesis (haem, chlorophyll, cobalamin etc.).
δ-Aminolevulinic acid: an intermediate in tetrapyrrole biosynthesis (haem, chlorophyll, cobalamin etc.).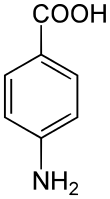 4-Aminobenzoic acid (PABA): an intermediate in folate biosynthesis
4-Aminobenzoic acid (PABA): an intermediate in folate biosynthesis
The reason why α-amino acids are used in proteins has been linked to their frequency in meteorites and prebiotic experiments.[7] An initial speculation on the deleterious properties of β-amino acids in terms of secondary structure,[7] turned out to be incorrect.[8]
D-amino acids
Some amino acids contain the opposite absolute chirality, chemicals that are not available from normal ribosomal translation/transcription machinery. Most bacterial cells walls are formed by peptidoglycan, a polymer composed of amino sugars crosslinked with short oligopeptides bridged between each other. The oligopeptide is non-ribosomally synthesised and contains several peculiarities including D-amino acids, generally D-alanine and D-glutamate. A further peculiarity is that the former is racemised by a PLP-binding enzymes (encoded by alr or the homologue dadX), whereas the latter is racemised by a cofactor independent enzyme (murI). Some variants are present, in Thermotoga spp. D-lysine is present and in certain vancomycin-resistant bacteria D-serine is present (vanT gene).[9] [10]
In animals, some D-amino acids are neurotransmitters.
Without a hydrogen on the α-carbon
All proteinogenic amino acids have at least one hydrogen on the α-carbon. Glycine has two hydrogens, and all others have one hydrogen and one side-chain. Replacement of the remaining hydrogen with a larger substituent, such as a methyl group, distorts the protein backbone.[7]
In some fungi α-amino isobutyric acid is produced as a precursor to peptides, some of which exhibit antibiotic properties.[11] This compound is similar to alanine, but possesses an additional methyl group on the α-carbon instead of a hydrogen. It is therefore achiral. Another compound similar to alanine without an α-hydrogen is dehydroalanine, which possess a methylene sidechain. It is one of several naturally occurring dehydroamino acids.
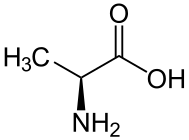 alanine
alanine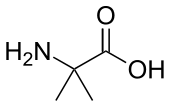 aminoisobutyric acid
aminoisobutyric acid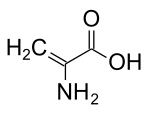 dehydroalanine
dehydroalanine
Twin amino acid stereocentres
A subset of L-α-amino acids are ambiguous as to which of two ends is the α-carbon. In proteins a cysteine residue can form a disulfide bond with another cysteine residue, thus crosslinking the protein. Two crosslinked cysteines form a cystine molecule. Cysteine and methionine are generally produced by direct sulfurylation, but in some species they can be produced by transsulfuration, where the activated homoserine or serine is fused to a cysteine or homocysteine forming cystathionine. A similar compound is lanthionine, which can be seen as two alanine molecules joined via a thioether bond and is found in various organisms. Similarly, djenkolic acid, a plant toxin from jengkol beans, is composed of two cysteines connected by a methylene group. Diaminopimelic acid is both used as a bridge in peptidoglycan and is used a precursor to lysine (via its decarboxylation).
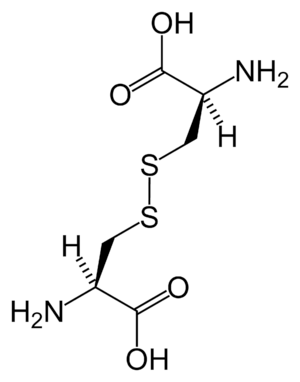 cystine
cystine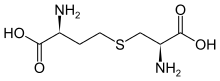 cystathionine
cystathionine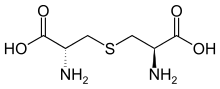 lanthionine
lanthionine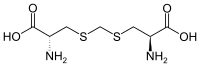 Djenkolic acid
Djenkolic acid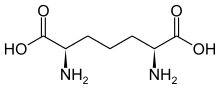 Diaminopimelic acid
Diaminopimelic acid
Prebiotic amino acids and alternative biochemistries
In meteorites and in prebiotic experiments (e.g. Miller–Urey experiment) many more amino acids than the twenty standard amino acids are found, several of which at higher concentrations than the standard ones: it has been conjectured that if amino acid based life were to arise in parallel elsewhere in the universe, no more than 75% of the amino acids would be in common.[7] The most notable anomaly is the lack of aminobutyric acid.
| Proportion of amino acids relative to glycine (%) | ||
|---|---|---|
| Molecule | Electric discharge | Murchinson meteorite |
| Glycine | 100 | 100 |
| Alanine | 180 | 36 |
| α-Amino-n-butyric acid | 61 | 19 |
| Norvaline | 14 | 14 |
| Valine | 4.4 | |
| Norleucine | 1.4 | |
| Leucine | 2.6 | |
| Isoleucine | 1.1 | |
| Alloisoleucine | 1.2 | |
| t-leucine | < 0.005 | |
| α-Amino-n-heptanoic acid | 0.3 | |
| Proline | 0.3 | 22 |
| Pipecolic acid | 0.01 | 11 |
| α,β-diaminopropionic acid | 1.5 | |
| α,γ-diaminobutyric acid | 7.6 | |
| Ornithine | < 0.01 | |
| lysine | < 0.01 | |
| Aspartic acid | 7.7 | 13 |
| Glutamic acid | 1.7 | 20 |
| Serine | 1.1 | |
| Threonine | 0.2 | |
| Allothreonine | 0.2 | |
| Methionine | 0.1 | |
| Homocysteine | 0.5 | |
| Homoserine | 0.5 | |
| β-Alanine | 4.3 | 10 |
| β-Amino-n-butyric acid | 0.1 | 5 |
| β-Aminoisobutyric acid | 0.5 | 7 |
| γ-Aminobutyric acid | 0.5 | 7 |
| α-Aminoisobutyric acid | 7 | 33 |
| isovaline | 1 | 11 |
| Sarcosine | 12.5 | 7 |
| N-ethyl glycine | 6.8 | 6 |
| N-propyl glycine | 0.5 | |
| N-isopropyl glycine | 0.5 | |
| N-methyl alanine | 3.4 | 3 |
| N-ethyl alanine | < 0.05 | |
| N-methyl β-alanine | 1.0 | |
| N-ethyl β-alanine | < 0.05 | |
| isoserine | 1.2 | |
| α-hydroxy-γ-aminobutyric acid | 17 |
Straight side chain
The genetic code has been described as a frozen accident and the reasons why there is only one standard amino acid with a straight chain (alanine) could simply be redundancy with valine, leucine and isoleucine.[7] However, straight chained amino acids are reported to form much more stable alpha helices.[12]
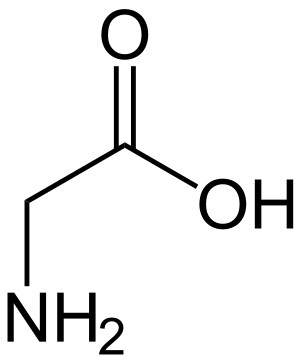 Glycine (Hydrogen side-chain)
Glycine (Hydrogen side-chain) Alanine (Methyl side-chain)
Alanine (Methyl side-chain)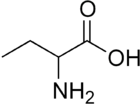 Homoalanine, or α-aminobutyric acid (Ethyl side-chain)
Homoalanine, or α-aminobutyric acid (Ethyl side-chain)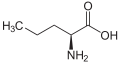 Norvaline (n-Propyl side-chain)
Norvaline (n-Propyl side-chain)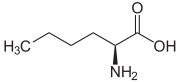 Norleucine (n-Butyl side-chain)
Norleucine (n-Butyl side-chain) Homonorleucine (n-Pentyl side-chain) (Heptanoic acid shown)
Homonorleucine (n-Pentyl side-chain) (Heptanoic acid shown)
Chalcogen
Serine, homoserine, O-methyl-homoserine and O-ethyl-homoserine possess an hydroxymethyl, hydroxyethyl, O-methyl-hydroxymethyl and O-methyl-hydroxyethyl side chain. Whereas cysteine, homocysteine, methionine and ethionine possess the thiol equivalents. The selenol equivalents are selenocysteine, selenohomocysteine, selenomethionine and selenoethionine. Amino acids with the next chalcogen down are also found in nature: several species such as Aspergillus fumigatus, Aspergillus terreus, and Penicillium chrysogenum in the absence of sulfur are able to produce and incorporate into protein tellurocysteine and telluromethionine.[13]
Hydroxyglycine, an amino acid with a hydroxyl side-chain, is highly unstable
Expanded genetic code
Roles
In cells, especially autotrophs, several non-proteinogenic amino acids are found as metabolic intermediates. However, despite the catalytic flexibility of PLP-binding enzymes, many amino acids are synthesised as keto-acids (e.g. 4-methyl-2-oxopentanoate to leucine) and aminated in the last step, thus keeping the number of non-proteinogenic amino acid intermediates fairly low.
Ornithine and citrulline occur in the urea cycle, part of amino acid catabolism (see below).[14]
In addition to primary metabolism, several non-proteinogenic amino acids are precursors or the final production in secondary metabolism to make small compounds or non-ribosomal peptides (such as some toxins).
Post-translationally incorporated into protein
Despite not being encoded by the genetic code as proteinogenic amino acids, some non-standard amino acids are nevertheless found in proteins. These are formed by post-translational modification of the side chains of standard amino acids present in the target protein. These modifications are often essential for the function or regulation of a protein; for example, in Gamma-carboxyglutamate the carboxylation of glutamate allows for better binding of calcium cations,[15] and in hydroxyproline the hydroxylation of proline is critical for maintaining connective tissues.[16] Another example is the formation of hypusine in the translation initiation factor EIF5A, through modification of a lysine residue.[17] Such modifications can also determine the localization of the protein, e.g., the addition of long hydrophobic groups can cause a protein to bind to a phospholipid membrane.[18]
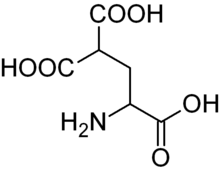 Carboxyglutamic acid. Whereas glutamic acid possess one γ-carboxyl group, Carboxyglutamic acid possess two.
Carboxyglutamic acid. Whereas glutamic acid possess one γ-carboxyl group, Carboxyglutamic acid possess two.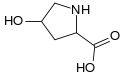 Hydroxyproline. This imino acid differs from proline due to a hydroxyl group on carbon 4.
Hydroxyproline. This imino acid differs from proline due to a hydroxyl group on carbon 4.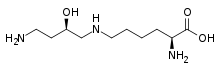 Hypusine. This amino acid is obtained by adding to the ε-amino group of a lysine a 4-aminobutyl moiety (obtained from spermidine)
Hypusine. This amino acid is obtained by adding to the ε-amino group of a lysine a 4-aminobutyl moiety (obtained from spermidine)-Pyroglutamic_acid_Structural_Formulae.png)
There is some preliminary evidence that aminomalonic acid may be present, possibly by misincorporation, in protein.[19][20]
Toxic analogues
Several non-proteinogenic amino acids are toxic due to their ability to mimic certain properties of proteinogenic amino acids, such as thialysine. Some non-proteinogenic amino acids are neurotoxic by mimicking amino acids used as neurotransmitters (i.e. not for protein biosynthesis), e.g. Quisqualic acid, canavanine or azetidine-2-carboxylic acid.[21] Cephalosporin C has an α-aminoadipic acid (homoglutamate) backbone that is amidated with a cephalosporin moiety.[22] Penicillamine is a therapeutic amino acid, whose mode of action is unknown.
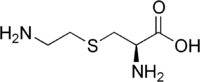 Thialysine
Thialysine Quisqualic acid
Quisqualic acid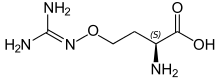 Canavanine
Canavanine-Azetidine-2-carboxylate.png) azetidine-2-carboxylic acid
azetidine-2-carboxylic acid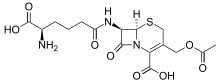 Cephalosporin C
Cephalosporin C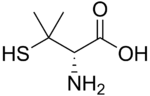 Penicillamine
Penicillamine
Naturally-occurring cyanotoxins can also include non-proteinogenic amino acids. Microcystin and nodularin, for example, are both derived from ADDA, a β-amino acid.
Not amino acids
Taurine is an amino sulfonic acid and not an amino acid, however it is occasionally considered as such as the amounts required to suppress the auxotroph in certain organisms (e.g. cats) are closer to those of "essential amino acids" (amino acid auxotrophy) than of vitamins (cofactor auxotrophy).
The osmolytes, sarcosine and glycine betaine are derived from amino acids, but have a secondary and quaternary amine respectively.
Notes
- plus formylmethionine in eukaryotes with prokaryote organelles like mitochondria
References
- Ambrogelly, A.; Palioura, S.; Söll, D. (2007). "Natural expansion of the genetic code". Nature Chemical Biology. 3 (1): 29–35. doi:10.1038/nchembio847. PMID 17173027.
- Böck, A.; Forchhammer, K.; Heider, J.; Baron, C. (1991). "Selenoprotein synthesis: An expansion of the genetic code". Trends in Biochemical Sciences. 16 (12): 463–467. doi:10.1016/0968-0004(91)90180-4. PMID 1838215.
- Théobald-Dietrich, A.; Giegé, R.; Rudinger-Thirion, J. L. (2005). "Evidence for the existence in mRNAs of a hairpin element responsible for ribosome dependent pyrrolysine insertion into proteins". Biochimie. 87 (9–10): 813–817. doi:10.1016/j.biochi.2005.03.006. PMID 16164991.
- Lu, Y.; Freeland, S. (2006). "On the evolution of the standard amino-acid alphabet". Genome Biology. 7 (1): 102. doi:10.1186/gb-2006-7-1-102. PMC 1431706. PMID 16515719.
- Voet, D.; Voet, J. G. (2004). Biochemistry (3rd ed.). John Wiley & Sons. ISBN 978-0471193500.
- Chakauya, E.; Coxon, K. M.; Ottenhof, H. H.; Whitney, H. M.; Blundell, T. L.; Abell, C.; Smith, A. G. (2005). "Pantothenate biosynthesis in higher plants". Biochemical Society Transactions. 33 (4): 743–746. doi:10.1042/BST0330743. PMID 16042590.
- Weber, A. L.; Miller, S. L. (1981). "Reasons for the occurrence of the twenty coded protein amino acids". Journal of Molecular Evolution. 17 (5): 273–284. Bibcode:1981JMolE..17..273W. doi:10.1007/BF01795749. PMID 7277510.
- Koyack, M. J.; Cheng, R. P. (2006). "Design and Synthesis of β-Peptides With Biological Activity". Protein Design. Methods in Molecular Biology. 340. pp. 95–109. doi:10.1385/1-59745-116-9:95. ISBN 978-1-59745-116-1. PMID 16957334.
- Boniface, A.; Parquet, C.; Arthur, M.; Mengin-Lecreulx, D.; Blanot, D. (2009). "The Elucidation of the Structure of Thermotoga maritima Peptidoglycan Reveals Two Novel Types of Cross-link". Journal of Biological Chemistry. 284 (33): 21856–21862. doi:10.1074/jbc.M109.034363. PMC 2755910. PMID 19542229.
- Arias, C. A.; Martín-Martinez, M.; Blundell, T. L.; Arthur, M.; Courvalin, P.; Reynolds, P. E. (1999). "Characterization and modelling of VanT: A novel, membrane-bound, serine racemase from vancomycin-resistant Enterococcus gallinarum BM4174". Molecular Microbiology. 31 (6): 1653–1664. doi:10.1046/j.1365-2958.1999.01294.x. PMID 10209740.
- Gao, X.; Chooi, Y. H.; Ames, B. D.; Wang, P.; Walsh, C. T.; Tang, Y. (2011). "Fungal Indole Alkaloid Biosynthesis: Genetic and Biochemical Investigation of the Tryptoquialanine Pathway inPenicillium aethiopicum". Journal of the American Chemical Society. 133 (8): 2729–2741. doi:10.1021/ja1101085. PMC 3045477. PMID 21299212.
- Padmanabhan, S.; Baldwin, R. L. (1991). "Straight-chain non-polar amino acids are good helix-formers in water". Journal of Molecular Biology. 219 (2): 135–137. doi:10.1016/0022-2836(91)90553-I. PMID 2038048.
- Ramadan, S. E.; Razak, A. A.; Ragab, A. M.; El-Meleigy, M. (1989). "Incorporation of tellurium into amino acids and proteins in a tellurium-tolerant fungi". Biological Trace Element Research. 20 (3): 225–232. doi:10.1007/BF02917437. PMID 2484755.
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. (2005). "Almost all about citrulline in mammals". Amino Acids. 29 (3): 177–205. doi:10.1007/s00726-005-0235-4. PMID 16082501.
- Vermeer, C. (1990). "Gamma-carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase". The Biochemical Journal. 266 (3): 625–636. doi:10.1042/bj2660625. PMC 1131186. PMID 2183788.
- Bhattacharjee, A; Bansal, M (2005). "Collagen structure: The Madras triple helix and the current scenario". IUBMB Life. 57 (3): 161–72. doi:10.1080/15216540500090710. PMID 16036578.
- Park, M. H. (2006). "The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A)". Journal of Biochemistry. 139 (2): 161–9. doi:10.1093/jb/mvj034. PMC 2494880. PMID 16452303.
- Blenis, J; Resh, M. D. (1993). "Subcellular localization specified by protein acylation and phosphorylation". Current Opinion in Cell Biology. 5 (6): 984–9. doi:10.1016/0955-0674(93)90081-z. PMID 8129952.
- Copley, S. D.; Frank, E.; Kirsch, W. M.; Koch, T. H. (1992). "Detection and possible origins of aminomalonic acid in protein hydrolysates". Analytical Biochemistry. 201 (1): 152–157. doi:10.1016/0003-2697(92)90188-D. PMID 1621954.
- Van Buskirk, J. J.; Kirsch, W. M.; Kleyer, D. L.; Barkley, R. M.; Koch, T. H. (1984). "Aminomalonic acid: Identification in Escherichia coli and atherosclerotic plaque". Proceedings of the National Academy of Sciences of the United States of America. 81 (3): 722–725. Bibcode:1984PNAS...81..722V. doi:10.1073/pnas.81.3.722. PMC 344907. PMID 6366787.
- Dasuri, K.; Ebenezer, P. J.; Uranga, R. M.; Gavilán, E.; Zhang, L.; Fernandez-Kim, S. O. K.; Bruce-Keller, A. J.; Keller, J. N. (2011). "Amino acid analog toxicity in primary rat neuronal and astrocyte cultures: Implications for protein misfolding and TDP-43 regulation". Journal of Neuroscience Research. 89 (9): 1471–1477. doi:10.1002/jnr.22677. PMC 3175609. PMID 21608013.
- Trown, P. W.; Smith, B.; Abraham, E. P. (1963). "Biosynthesis of cephalosporin C from amino acids". The Biochemical Journal. 86 (2): 284–291. doi:10.1042/bj0860284. PMC 1201751. PMID 13994319.