Pipecolic acid
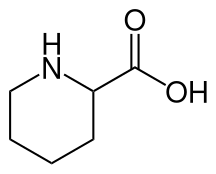
Pipecolic acid (piperidine-2-carboxylic acid) is a small organic molecule which accumulates in pipecolic acidemia. It is a carboxylic acid of piperidine.
 | |
| Names | |
|---|---|
| IUPAC name
piperidine-2-carboxylic acid | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.835 |
| EC Number |
|
| KEGG | |
| MeSH | C031345 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H11NO2 | |
| Molar mass | 129.15704 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biochemical effects
Pipecolic acid can be associated with some forms of epilepsy.[1]
CRYM, a taxon-specific crystallin protein that also binds thyroid hormones, is involved in the pipecolic acid pathway.
Environmental abundance and relevance
Pipecolic acid was identified in the Murchison meteorite.[2] It also occurs in the leaves of the genus Myroxylon, a tree from South America.[3]
Stereochemistry
Pipecolic acid, like many other α-amino acids, has a chiral stereocenter at the α carbon.
gollark: Suuuure.
gollark: It's already been sent to our role launderers.
gollark: Stealing your role obviously.
gollark: <@319753218592866315>
gollark: That is available with the -XMetacomonadicTreestructures extension.
See also
References
- Plecko B, Hikel C, Korenke GC, et al. (2005). "Pipecolic acid as a diagnostic marker of pyridoxine-dependent epilepsy". Neuropediatrics. 36 (3): 200–5. doi:10.1055/s-2005-865727. PMID 15944906.
- Kvenholden, Keith A.; Lawless, James G.; Ponnamperuma, Cyril (February 1971). "Nonprotein Amino Acids in the Murchison Meteorite". Proceedings of the National Academy of Sciences. 68 (2): 486–490. Bibcode:1971PNAS...68..486K. doi:10.1073/pnas.68.2.486. PMC 388966. PMID 16591908.
- Kite, GC; Cardoso, D; Lewis, GP; Zartman, CE; de Queiroz, LP; Veitch, NC (2015). "Monomethyl ethers of 4,5-dihydroxypipecolic acid from Petaladenium urceoliferum: Enigmatic chemistry of an enigmatic legume". Phytochemistry. 116: 198–202. doi:10.1016/j.phytochem.2015.02.026. PMID 25817832.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.