Tellurocysteine
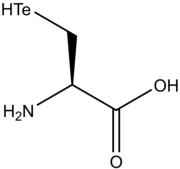
Tellurocysteine (in some publications referred to as Te-Cys) is an amino acid analogous to serine, cysteine and selenocysteine with tellurium in place of oxygen, sulfur or selenium in its side chain. It is not naturally found in organisms.
 | |
| Names | |
|---|---|
| IUPAC name
(2R)-2-Amino-3-tellanylpropanoic acid | |
| Other names
Tellurocystine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C3H7NO2Te | |
| Molar mass | 216.69 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties
Perhaps due to its low bond energy with carbon (200 kJ/mol compared to 234 kJ/mol for selenium, or 272 kJ/mol for sulfur),[1] tellurocysteine is not present in any known natural organisms and is hence relatively understudied in comparison to selenocysteine.[2] Despite so, certain organisms such as fungi Aspergillus fumigatus is capable of incorporating tellurocysteine and telluromethionine into amino acids and proteins when exposed to a sulfur-free environment.[3]
It has been observed that when incorporated into glutathione transferase, tellurocysteine efficiently inhibited aminoacylation and increased the efficiency of glutathione peroxidase.[4]
Synthesis
L-Tellurocysteine has been prepared from elemental tellurium by first reacting it with methyl (2R)-2-[(tert-butoxycarbonyl)amino]-3-iodopropionate in a tetrahydrofuran solvent with presence of lithium triethylborohydride, yielding a red oil which is acidified and treated with alkali before a treatment with citric acid to set its pH to 4.0, resulting in an orange solid after filtering and drying.[5]
References
- Chivers, Tristram; Laitinen, Risto S. (23 March 2015). "Tellurium: a maverick among the chalcogens". Chemical Society Reviews. 44 (7): 1725–1739. doi:10.1039/C4CS00434E. ISSN 1460-4744. PMID 25692398.
- Advances in Microbial Physiology. Academic Press. 2007. p. 4. ISBN 9780080560649.
- Ramadan, ShadiaE.; Razak, A.A.; Ragab, A.M.; El-Meleigy, M. (1 June 1989). "Incorporation of tellurium into amino acids and proteins in a tellurium-tolerant fungi". Biological Trace Element Research. 20 (3): 225–232. doi:10.1007/BF02917437. ISSN 0163-4984. PMID 2484755.
- Liu, Xiaoman; Silks, Louis A.; Liu, Cuiping; Ollivault-Shiflett, Morgane; Huang, Xin; Li, Jing; Luo, Guimin; Hou, Ya-Ming; Liu, Junqiu; Shen, Jiacong (2 March 2009). "Incorporation of Tellurocysteine into Glutathione Transferase Generates High Glutathione Peroxidase Efficiency". Angewandte Chemie International Edition. 48 (11): 2020–2023. doi:10.1002/anie.200805365. PMID 19199319.
- Stocking, Emily M.; Schwarz, Jessie N.; Senn, Hans; Salzmann, Michael; Silks, Louis A. (1 January 1997). "Synthesis of L-selenocystine,L-[77Se]selenocystine andL-tellurocystine". Journal of the Chemical Society, Perkin Transactions 1. 0 (16): 2443–2448. doi:10.1039/A600180G. ISSN 1364-5463.