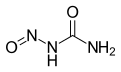
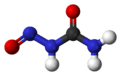
Nitrosourea
Nitrosourea is both the name of a molecule, and a class of compounds that include a nitroso (R-NO) group and a urea.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Nitrosourea | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| CH3N3O2 | |||
| Molar mass | 89.054 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Examples
Examples include:
- Arabinopyranosyl-N-methyl-N-nitrosourea (Aranose)
- Carmustine (BCNU, BiCNU)
- Chlorozotocin
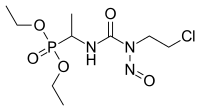
- Ethylnitrosourea (ENU)
- Fotemustine
- Lomustine (CCNU)
- Nimustine
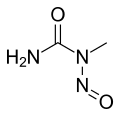
- N-Nitroso-N-methylurea (NMU)
- Ranimustine (MCNU)
- Semustine
- Streptozocin (Streptozotocin)
Nitrosourea compounds are DNA alkylating agents and are often used in chemotherapy.[1] They are lipophilic and thus can cross the blood–brain barrier, making them useful in the treatment of brain tumors such as glioblastoma multiforme.[2]
Side effects
Some nitrosoureas (e.g. lomustine) have been associated with the development of interstitial lung disease.[3]
gollark: This ENTIRE SYSTEM was designed WRONGLY.
gollark: Aha, it looks like I managed to poke the USB daemon into doing certain things or something.
gollark: Wow, I managed to crash the entire init system, fun.
gollark: It doesn't actually have vim either.
gollark: Oh hypermemetic beeoids, what sort of horrible system doesn't even have *nano*?
References
- "Antineop". Archived from the original on 2009-03-07. Retrieved 2009-01-24.
- Takimoto CH, Calvo E. "Principles of oncologic pharmacotherapy". in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer management: a multidisciplinary approach. 11 ed. 2008.
- Tucci E, Verdiani P, Di Carlo S, Sforza V (1986). "Lomustine (CCNU)-induced pulmonary fibrosis". Tumori. 72 (1): 95–8. PMID 3952821.
External links
- Nitrosourea+Compounds at the US National Library of Medicine Medical Subject Headings (MeSH)
- DDB 9052
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
.svg.png)
.svg.png)
.svg.png)
.svg.png)