Metallacyclopentanes
In organometallic chemistry, metallacyclopentanes are compounds with the formula LnM(CH2)4 (Ln = ligands, and M = metal). They are a type of metallacycle. Metallacyclopentanes are intermediates in some metal-catalysed reactions in homogeneous catalysis.
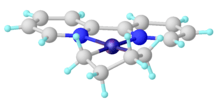
Synthesis and structure
Traditionally, metallacyclopentanes are prepared by dialkylation of metal dihalides with 1,4‐bis(bromomagnesio)butane or the related dilithio reagent.[2] The complex Ni(bipyridine)C4H8 is prepared by oxidative addition of 1,4-dibromobutane to Ni(0) precursors.[1] Metallacyclopentanes also arise via the dimerization of ethylene within the coordination sphere of a low-valence metal center. This reaction is relevant to the catalytic production of butenes and related alkenes.
Occurrence
Early examples of metallacyclopentanes come from studies of the Ni-catalyzed linear- and cyclo-dimerization of ethylenes. Linear dimerization proceeds via beta-hydride elimination of the nickelacyclopentane (Ph3P)Ni(CH2)4 whereas cyclodimerization to give cyclobutane proceeds by reductive elimination from the related (Ph3P)2Ni(CH2)4.[3] Another example of a metallacyclopentane is the titanocene derivative Cp2Ti(CH2)4.[4]
Metallacyclopentanes are intermediates in the metal-catalysed dimerization, trimerization, and tetramerization of ethylene to give 1-butene, 1-hexene, and 1-octene, respectively. These compounds are of commercial interest as comonomers, used in the production of polyethylene.[5]
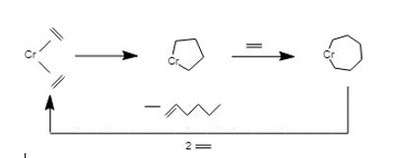
In the evolution of heterogeneous alkene metathesis catalysts, metallacyclopentanes are invoked as intermediates in the formation of metal alkylidenes from ethylene. Thus, metallacyclopentane intermediates are proposed to isomerize to metallacyclobutanes, which can eliminate alkene giving the alkylidene.[6]
Structure
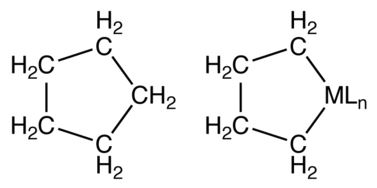
Unsubstituted metallacyclopentanes adopt conformations related to cyclopentane itself: open-envelope conformation and a twisted open-envelope structure.[7]
See also
References
- P. Binger, M. J. Doyle, C. Kruger, Y.-H. Tsay (1979). "Darstellung und Characterisierung von α,α'-Bipyridyl-Nickelacyclopentan (Preparation and Characterization of α,α'-Bipyridyl-Nickelacyclopentane)". Z. Naturforsch. B. 34: 1289. doi:10.1515/znb-1979-0926.CS1 maint: uses authors parameter (link)
- Perséphone Canonne, Paul Angers (2001). "1,4-Bis(bromomagnesio)butane". 1,4‐Bis(bromomagnesio)butane. Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rb109s. ISBN 0471936235.CS1 maint: uses authors parameter (link)
- Grubbs, Robert H.; Miyashita, Akira (November 1978). "Metallacyclopentanes as catalysts for the linear and cyclodimerization of olefins". Journal of the American Chemical Society. 100 (23): 7416–7418. doi:10.1021/ja00491a051.
- Stockis, Armel; Hoffmann, Roald (April 1980). "Metallacyclopentanes and bisolefin complexes". Journal of the American Chemical Society. 102 (9): 2952–2962. doi:10.1021/ja00529a015.
- Dixon, John T.; Green, Mike J. (15 November 2004). "Advances in Selective Ethylene Trimerisation - a critical review". Journal of Organometallic Chemistry. 689 (23): 3641–3668. doi:10.1016/j.jorganchem.2004.06.008.
- Schrock, R. R.; Coperet, C. (2017). "Formation of High-Oxidation-State Metal-Carbon Double Bonds". Organometallics. 36: 1884–1892. doi:10.1021/acs.organomet.6b00825. hdl:1721.1/115114.CS1 maint: uses authors parameter (link)
- Lindner, Ekkehard (1986). "Metallacycloalkanes and -alkenes". Advances in Heterocyclic Chemistry. 39: 237–279. doi:10.1016/S0065-2725(17)30059-4.