Ion exchange
Ion exchange usually describes a processes of purification of aqueous solutions using solid polymeric ion exchange resin. More precisely, the term encompasses a large variety of processes where ions are exchanged between two electrolytes.[1] Aside from its use to purify drinking water, the technique is widely applied for purification and separation of a variety of industrially and medicinally important chemicals. Although the term usually refers to applications of synthetic (man-made) resins, many materials, in particular soil.


Typical ion exchangers are ion-exchange resins (functionalized porous or gel polymer), zeolites, montmorillonite, clay, and soil humus. Ion exchangers are either cation exchangers, which exchange positively charged ions (cations), or anion exchangers, which exchange negatively charged ions (anions). There are also amphoteric exchangers that are able to exchange both cations and anions simultaneously. However, the simultaneous exchange of cations and anions can be more efficiently performed in mixed beds, which contain a mixture of anion- and cation-exchange resins, or passing the treated solution through several different ion-exchange materials.

Ion exchanges can be unselective or have binding preferences for certain ions or classes of ions, depending on their chemical structure. This can be dependent on the size of the ions, their charge, or their structure. Typical examples of ions that can bind to ion exchangers are:
- H+ (proton) and OH− (hydroxide).
- Singly charged monatomic ions like Na+, K+, and Cl−.
- Doubly charged monatomic ions like Ca2+ and Mg2+.
- Polyatomic inorganic ions like SO42− and PO43−.
- Organic bases, usually molecules containing the amine functional group −NR2H+.
- Organic acids, often molecules containing −COO− (carboxylic acid) functional groups.
- Biomolecules that can be ionized: amino acids, peptides, proteins, etc.
Along with absorption and adsorption, ion exchange is a form of sorption.
Ion exchange is a reversible process, and the ion exchanger can be regenerated or loaded with desirable ions by washing with an excess of these ions.
Applications
Ion exchange is widely used in the food and beverage industry, hydrometallurgy, metals finishing, chemical, petrochemical, pharmaceutical technology, sugar and sweetener production, ground- and potable-water treatment, nuclear, softening, industrial water treatment, semiconductor, power, and many other industries.
A typical example of application is preparation of high-purity water for power engineering, electronic and nuclear industries; i.e. polymeric or mineralic insoluble ion exchangers are widely used for water softening, water purification,[2] water decontamination, etc.
Ion exchange is a method widely used in household (laundry detergents and water filters) to produce soft water. This is accomplished by exchanging calcium Ca2+ and magnesium Mg2+ cations against Na+ or H+ cations (see water softening). Another application for ion exchange in domestic water treatment is the removal of nitrate and natural organic matter.
Industrial and analytical ion-exchange chromatography is another area to be mentioned. Ion-exchange chromatography is a chromatographical method that is widely used for chemical analysis and separation of ions. For example, in biochemistry it is widely used to separate charged molecules such as proteins. An important area of the application is extraction and purification of biologically produced substances such as proteins (amino acids) and DNA/RNA.
Ion-exchange processes are used to separate and purify metals, including separating uranium from plutonium and the other actinides, including thorium, neptunium, and americium. This process is also used to separate the lanthanides, such as lanthanum, cerium, neodymium, praseodymium, europium, and ytterbium, from each other. The separation of neodymium and praseodymium was a particularly difficult one, and those were formerly thought to be just one element didymium - but that is an alloy of the two.
There are two series of rare-earth metals, the lanthanides and the actinides, both of whose families all have very similar chemical and physical properties. Using methods developed by Frank Spedding in the 1940s, ion exchange processes were formerly the only practical way to separate them in large quantities, until the development of the "solvent extraction" techniques that can be scaled up enormously..
A very important case of ion-exchange is the PUREX process (Plutonium-URanium Extraction Process), which is used to separate the plutonium-239 and the uranium from americium, curium, neptunium, the radioactive fission products that come from nuclear reactors. Thus the waste products can be separated out for disposal. Next, the plutonium and uranium are available for making nuclear-energy materials, such as new reactor fuel and nuclear weapons.
The ion-exchange process is also used to separate other sets of very similar chemical elements, such as zirconium and hafnium, which is also very important for the nuclear industry. Physically, zirconium is practically transparent to free neutrons, used in building nuclear reactors, but hafnium is a very strong absorber of neutrons, used in reactor control rods. Thus, ion-exchange is used in nuclear reprocessing and the treatment of radioactive waste.
Ion-exchange resins in the form of thin membranes are also used in chloralkali process, fuel cells, and vanadium redox batteries.
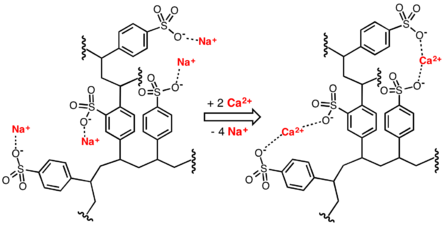

Ion exchange can also be used to remove hardness from water by exchanging calcium and magnesium ions for sodium ions in an ion-exchange column. Liquid-phase (aqueous) ion-exchange desalination has been demonstrated.[4] In this technique anions and cations in salt water are exchanged for carbonate anions and calcium cations respectively using electrophoresis. Calcium and carbonate ions then react to form calcium carbonate, which then precipitates, leaving behind fresh water. The desalination occurs at ambient temperature and pressure and requires no membranes or solid ion exchangers. The theoretical energy efficiency of this method is on par with electrodialysis and reverse osmosis.
Other applications
- In soil science, cation-exchange capacity is the ion-exchange capacity of soil for positively charged ions. Soils can be considered as natural weak cation exchangers.
- In pollution remediation and geotechnical engineering, ion-exchange capacity determines the swelling capacity of swelling or expansive clay such as montmorillonite, which can be used to "capture" pollutants and charged ions.
- In planar waveguide manufacturing, ion exchange is used to create the guiding layer of higher index of refraction.
- Dealkalization, removal of alkali ions from a glass surface.
- Chemically strengthened glass, produced by exchanging K+ for Na+ in soda glass surfaces using KNO3 melts.
Regenerating wasted water
Most ion-exchange systems contain containers of ion-exchange resin that are operated on a cyclic basis.
During the filtration process, water flows through the resin container until the resin is considered exhausted. That happens only when water leaving the exchanger contains more than the maximal desired concentration of the ions being removed. Resin is then regenerated by sequentially backwashing the resin bed to remove accumulated solids, flushing removed ions from the resin with a concentrated solution of replacement ions, and rinsing the flushing solution from the resin. Production of backwash, flushing, and rinsing wastewater during regeneration of ion-exchange media limits the usefulness of ion exchange for wastewater treatment.[5]
Water softeners are usually regenerated with brine containing 10% sodium chloride.[6] Aside from the soluble chloride salts of divalent cations removed from the softened water, softener regeneration wastewater contains the unused 50 – 70% of the sodium chloride regeneration flushing brine required to reverse ion-exchange resin equilibria. Deionizing resin regeneration with sulfuric acid and sodium hydroxide is approximately 20–40% efficient. Neutralized deionizer regeneration wastewater contains all of the removed ions plus 2.5–5 times their equivalent concentration as sodium sulfate.[7]
Further information
- Betz Laboratories (1976). Handbook of Industrial Water Conditioning (7th Edition). Betz Laboratories.
- Ion Exchangers (K. Dorfner, ed.), Walter de Gruyter, Berlin, 1991.
- C. E. Harland, Ion exchange: Theory and Practice, The Royal Society of Chemistry, Cambridge, 1994.
- Friedrich G. Helfferich (1962). Ion Exchange. Courier Dover Publications. ISBN 978-0-486-68784-1.
- Kemmer, Frank N. (1979). The NALCO Water Handbook. McGraw-Hill.
- Ion exchange (D. Muraviev, V. Gorshkov, A. Warshawsky), M. Dekker, New York, 2000.
- A. A. Zagorodni, Ion Exchange Materials: Properties and Applications, Elsevier, Amsterdam, 2006.
- Illustrated and well defined chemistry lab practical on ion exchange from Dartmouth College
- Some applets illustrating ion exchange processes
- A simple explanation of deionization
- Ion exchange, BioMineWiki
See also
- Alkali anion exchange membrane
- Ion
- Ion chromatography
- Ion-exchange membranes
- Ion-exchange resin
- Desalination
References
- Dardel, François; Arden, Thomas V. (2008). "Ion Exchangers". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a14_393.pub2.
- Ibrahim, Yazan; Abdulkarem, Elham; Naddeo, Vincenzo; Banat, Fawzi; Hasan, Shadi W. (November 2019). "Synthesis of super hydrophilic cellulose-alpha zirconium phosphate ion exchange membrane via surface coating for the removal of heavy metals from wastewater". Science of the Total Environment. 690: 167–180. Bibcode:2019ScTEn.690..167I. doi:10.1016/j.scitotenv.2019.07.009. PMID 31288108.
- Mischissin, Stephen G. (7 February 2012). "University of Rochester - Investigation of Steam Turbine Extraction Line Failures" (PDF). Arlington, VA. pp. 25–26. Archived from the original (PDF) on 23 September 2015. Retrieved 23 February 2015.
- Shkolnikov, Viktor; Bahga, Supreet S.; Santiago, Juan G. (August 28, 2012). "Desalination and hydrogen, chlorine, and sodium hydroxide production via electrophoretic ion exchange and precipitation" (PDF). Physical Chemistry Chemical Physics. Phys. Chem. Chem Phys. 14 (32): 11534–45. Bibcode:2012PCCP...1411534S. doi:10.1039/c2cp42121f. PMID 22806549.
- Kemmer, pp. 12–17, 12 – 25.
- Betz, p. 59.
- Kemmer, p. 12 – 18.
External links
| Wikimedia Commons has media related to Ion exchange. |
This method is also named as permutit (or)
{Wastewater}}