PUREX
PUREX (plutonium uranium reduction extraction) is a chemical method used to purify fuel for nuclear reactors or nuclear weapons.[1] PUREX is the de facto standard aqueous nuclear reprocessing method for the recovery of uranium and plutonium from used nuclear fuel (spent nuclear fuel, or irradiated nuclear fuel). It is based on liquid–liquid extraction ion-exchange.[2]
PUREX is applied to spent nuclear fuel, which consists primarily of very high atomic-weight (actinoid or "actinide") elements (e.g. uranium, plutonium, americium) along with smaller amounts of material composed of lighter atoms, notably the so-called fission products.
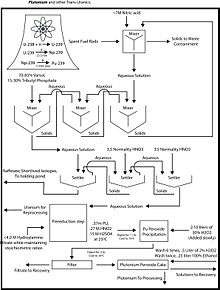
The actinoid elements in this case consist primarily of the unconsumed remains of the original fuel (typically U-235, U-238, and/or Pu-239).
Chemical process
The fuel is first dissolved in nitric acid at a concentration of ca. 7 M. Solids are removed by filtration lest they form emulsions, referred to as third phases in the solvent extraction community.
The organic solvent consists of 30% tributyl phosphate (TBP) in a hydrocarbon, such as kerosene. Uranium ions are extracted as UO2(NO3)2·2TBP complexes, and plutonium as similar complexes. Other fission products remain in the aqueous phase, including americium and curium. The nature of the organic soluble uranium complex has been the subject of some research. The nature of uranyl nitrate complexes with trialkyl phosphates have been characterized.[3]
Plutonium is separated from uranium by treating the kerosene solution with reducing agents to convert the plutonium to the +3 oxidation state. Typical reducing agents include N,N-diethyl-hydroxylamine, ferrous sulphamate, and hydrazine. The plutonium 3+ passes into the aqueous phase. The uranium is stripped from the kerosene solution by back-extraction into nitric acid at a concentration of ca. 0.2 M.[4]
PUREX raffinate
The term PUREX raffinate describes the mixture of metals in nitric acid which are left behind when the uranium and plutonium have been removed by the PUREX process from a nuclear fuel dissolution liquor. This mixture is often known as high level nuclear waste.
Two PUREX raffinates exist. The most highly active raffinate from the first cycle is the one which is most commonly known as PUREX raffinate. The other is from the medium-active cycle in which the uranium and plutonium are refined by a second extraction with tributyl phosphate.
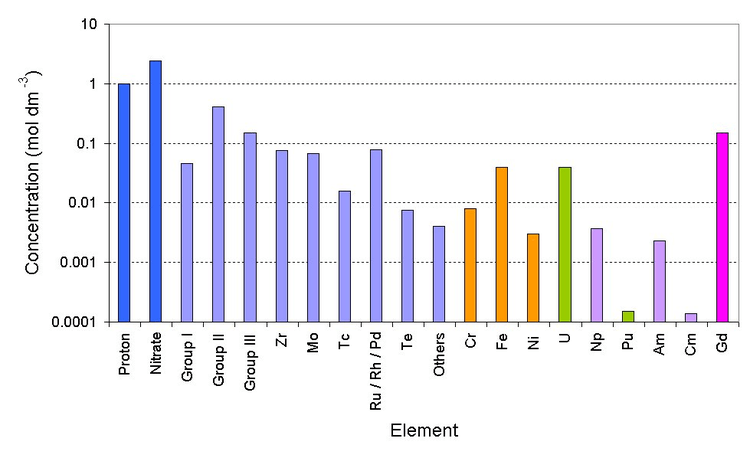
Deep blue is the bulk ions, light blue is the fission products (group I is Rb/Cs) (group II is Sr/Ba) (group III is Y and the lanthanides), orange is the corrosion products (from stainless steel pipework), green are the major actinides, violet are the minor actinides and magenta is the neutron poison)
Currently PUREX raffinate is stored in stainless steel tanks before being converted into glass. The first cycle PUREX raffinate is very radioactive. It has almost all of the fission products, corrosion products such as iron/nickel, traces of uranium, plutonium and the minor actinides.
Pollution
The PUREX Plant at the Hanford Site was responsible for producing 'copious volumes of liquid wastes', resulting in the radioactive contamination of groundwater.[5]
Greenpeace measurements in La Hague and Sellafield indicated that radioactive pollutants are steadily released into the sea, and the air. Therefore, people living near these processing plants are exposed to higher radiation levels than the naturally occurring background radiation. This additional radiation is small but not negligible according to Greenpeace.[6]
History
The PUREX process was invented by Herbert H. Anderson and Larned B. Asprey at the Metallurgical Laboratory at the University of Chicago, as part of the Manhattan Project under Glenn T. Seaborg; their patent "Solvent Extraction Process for Plutonium" filed in 1947,[7] mentions tributyl phosphate as the major reactant which accomplishes the bulk of the chemical extraction.[8]
List of nuclear reprocessing sites
- COGEMA La Hague site
- Mayak
- Thermal Oxide Reprocessing Plant and B205 at Sellafield
- Tokai, Ibaraki
- West Valley Reprocessing Plant
- Savannah River Site
- Hanford Site
- Idaho Chemical Processing Plant, (now Idaho National Laboratory)
- Radiochemical Engineering Development Center, Oak Ridge National Laboratory
See also
- Nuclear fuel cycle
- Nuclear breeder reactor
- Spent nuclear fuel shipping cask
- Global Nuclear Energy Partnership announced February, 2006
References & notes
- Gregory Choppin; Jan-Olov Liljenzin; Jan Rydberg (2002). Radiochemistry and Nuclear Chemistry, Third Edition. p. 610. ISBN 978-0-7506-7463-8.
- Paiva, A. P.; Malik, P. (2004). "Recent advances on the chemistry of solvent extraction applied to the reprocessing of spent nuclear fuels and radioactive wastes". Journal of Radioanalytical and Nuclear Chemistry. 261 (2): 485–496. doi:10.1023/B:JRNC.0000034890.23325.b5.CS1 maint: uses authors parameter (link)
- J.H. Burns (1983). "Solvent-extraction complexes of the uranyl ion. 2. Crystal and molecular structures of catena-bis(.mu.-di-n-butyl phosphato-O,O')dioxouranium(VI) and bis(.mu.-di-n-butyl phosphato-O,O')bis[(nitrato)(tri-n-butylphosphine oxide)dioxouranium(VI)]". Inorganic Chemistry. 22 (8): 1174–1178. doi:10.1021/ic00150a006.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 1261. ISBN 978-0-08-037941-8.
- Gerber, M.S. (February 2001). "History of Hanford Site Defense Production (Brief)" (PDF). Fluor Hanford / US DOE. Retrieved 2009-10-01.
- "Greenpeace on La Hague (German version)". Retrieved 2016-04-30.
- US patent 2924506, Anderson, Herbert H. and Asprey, Larned B. & Asprey, Larned B., "Solvent extraction process for plutonium", issued 1960-02-09
- P. Gary Eller; Bob Penneman & Bob Ryan (2005). "Pioneer actinide chemist Larned Asprey dies" (PDF). The Actinide Research Quarterly. Los Alamos National Laboratory. pp. 13–17. Archived from the original (PDF) on 2014-02-01.
Further reading
- OECD Nuclear Energy Agency, The Economics of the Nuclear Fuel Cycle, Paris, 1994
- I. Hensing and W Schultz, Economic Comparison of Nuclear Fuel Cycle Options, Energiewirtschaftlichen Instituts, Cologne, 1995.
- Cogema, Reprocessing-Recycling: the Industrial Stakes, presentation to the Konrad-Adenauer-Stiftung, Bonn, 9 May 1995.
- OECD Nuclear Energy Agency, Plutonium Fuel: An Assessment, Paris, 1989.
- National Research Council, "Nuclear Wastes: Technologies for Separation and Transmutation", National Academy Press, Washington D.C. 1996.
External links
- Processing of Used Nuclear Fuel, World Nuclear Association
- Reactor-Grade Plutonium and Development of Nuclear Weapons, Analytical Center for Non-proliferation
- PUREX Process, European Nuclear Society
- Mixed Oxide Fuel (MOX) – World Nuclear Association
- Disposal Options for Surplus Weapons-Usable Plutonium – Congressional Research Service Report for Congress
- Brief History of Fuel Reprocessing