Chloralkali process
The chloralkali process (also chlor-alkali and chlor alkali) is an industrial process for the electrolysis of sodium chloride solutions. It is the technology used to produce chlorine and sodium hydroxide (lye/caustic soda),[1] which are commodity chemicals required by industry. 35 million tons of chlorine were prepared by this process in 1987.[2] Industrial scale production began in 1892.
Usually the process is conducted on a brine (an aqueous solution of NaCl), in which case NaOH, hydrogen, and chlorine result. When using calcium chloride or potassium chloride, the products contain calcium or potassium instead of sodium. Related processes are known that use molten NaCl to give chlorine and sodium metal or condensed hydrogen chloride to give hydrogen and chlorine.
The process has a high energy consumption, for example around 2500 kWh of electricity per tonne of sodium hydroxide produced. Because the process yields equivalent amounts of chlorine and sodium hydroxide (two moles of sodium hydroxide per mole of chlorine), it is necessary to find a use for these products in the same proportion. For every mole of chlorine produced, one mole of hydrogen is produced. Much of this hydrogen is used to produce hydrochloric acid, ammonia, hydrogen peroxide, or is combusted for power and/or steam production.[3]
Process systems
Three production methods are in use. While the mercury cell method produces chlorine-free sodium hydroxide, the use of several tonnes of mercury leads to serious environmental problems. In a normal production cycle a few hundred pounds of mercury per year are emitted, which accumulate in the environment. Additionally, the chlorine and sodium hydroxide produced via the mercury-cell chloralkali process are themselves contaminated with trace amounts of mercury. The membrane and diaphragm method use no mercury, but the sodium hydroxide contains chlorine, which must be removed.
Membrane cell
The most common chloralkali process involves the electrolysis of aqueous sodium chloride (a brine) in a membrane cell. A membrane, such as one made from Nafion, Flemion or Aciplex, is used to prevent the reaction between the chlorine and hydroxide ions.
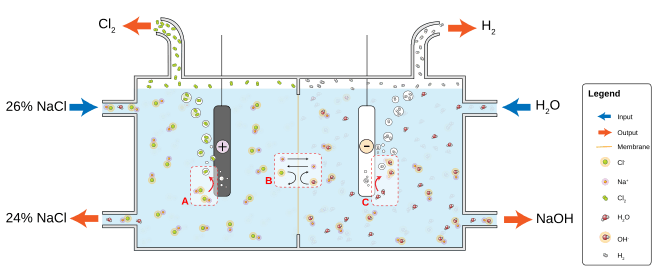
Saturated brine is passed into the first chamber of the cell where the chloride ions are oxidised at the anode, losing electrons to become chlorine gas (A in figure):
- 2Cl− → Cl
2 + 2e−
At the cathode, positive hydrogen ions pulled from water molecules are reduced by the electrons provided by the electrolytic current, to hydrogen gas, releasing hydroxide ions into the solution (C in figure):
- 2H
2O + 2e− → H2 + 2OH−
The ion-permeable ion exchange membrane at the center of the cell allows the sodium ions (Na+) to pass to the second chamber where they react with the hydroxide ions to produce caustic soda (NaOH) (B in figure).[1] The overall reaction for the electrolysis of brine is thus:
- 2NaCl + 2H
2O → Cl
2 + H
2 + 2NaOH
Diaphragm cell
In the diaphragm cell process, there are two compartments separated by a permeable diaphragm, often made of asbestos fibers. Brine is introduced into the anode compartment and flows into the cathode compartment. Similarly to the Membrane Cell, chloride ions are oxidized at the anode to produce chlorine, and at the cathode, water is split into caustic soda and hydrogen. The diaphragm prevents the reaction of the caustic soda with the chlorine. A diluted caustic brine leaves the cell. The caustic soda must usually be concentrated to 50% and the salt removed. This is done using an evaporative process with about three tonnes of steam per tonne of caustic soda. The salt separated from the caustic brine can be used to saturate diluted brine. The chlorine contains oxygen and must often be purified by liquefaction and evaporation.
Mercury cell
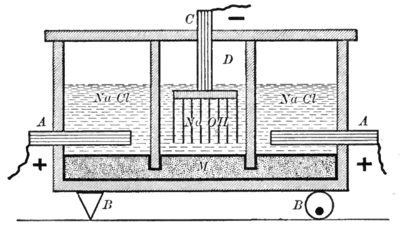
In the mercury-cell process, also known as the Castner–Kellner process, a saturated brine solution floats on top of a thin layer of mercury. The mercury is the cathode, where sodium is produced and forms a sodium-mercury amalgam with the mercury. The amalgam is continuously drawn out of the cell and reacted with water which decomposes the amalgam into sodium hydroxide, hydrogen and mercury. The mercury is recycled into the electrolytic cell. Chlorine is produced at the anode and bubbles out of the cell. Mercury cells are being phased out due to concerns about mercury poisoning from mercury cell pollution such as occurred in Canada (see Ontario Minamata disease) and Japan (see Minamata disease).
Unpartitioned cell
The initial overall reaction produces hydroxide and also hydrogen and chlorine gases:[4]
- 2 NaCl + 2 H2O → 2 NaOH + H2 + Cl2
Without a membrane, the OH− ions produced at the cathode are free to diffuse throughout the electrolyte. As the electrolyte becomes more basic due to the production of OH−, less Cl2 emerges from the solution as it begins to disproportionate to form chloride and hypochlorite ions at the anode:
- 2 Cl2 + NaOH → NaCl + NaClO + H2O
The more opportunity the Cl2 has to interact with NaOH in the solution, the less Cl2 emerges at the surface of the solution and the faster the production of hypochlorite progresses. This depends on factors such as solution temperature, the amount of time the Cl2 molecule is in contact with the solution, and concentration of NaOH.
Likewise, as hypochlorite increases in concentration, chlorates are produced from them:
- 3 NaClO → NaClO3 + 2 NaCl
This reaction is accelerated at temperatures above about 60 °C. Other reactions occur, such as the self-ionization of water and the decomposition of hypochlorite at the cathode, the rate of the latter depends on factors such as diffusion and the surface area of the cathode in contact with the electrolyte.[5]
If current is interrupted while the cathode is submerged, cathodes that are attacked by hypochlorites, such as those made from stainless steel, will dissolve in unpartitioned cells.
If producing hydrogen and oxygen gases is not a priority, the addition of 0.18% sodium or potassium chromate to the electrolyte will improve the efficiency of producing the other products.[5]
Electrodes
Due to the corrosive nature of chlorine production, the anode (where the chlorine is formed) must be non-reactive and has been made from platinum metal[6], graphite (called plumbago in Faraday's time)[6], platinized titanium.[7] A mixed metal oxide clad titanium anode (also called a dimensionally stable anode) is the industrial standard today. Historically, platinum, magnetite, lead dioxide[8], manganese dioxide, and ferrosilicon (13-15% silicon[9]) have also been used as anodes.[10] Platinum alloyed with iridium is more resistant to corrosion from chlorine than pure platinum.[10][11] Unclad titanium cannot be used as an anode because it anodizes, forming a non-conductive oxide and passivates. Graphite will slowly disintegrate due to internal electrolytic gas production from the porous nature of the material and carbon dioxide forming due to carbon oxidation, causing fine particles of graphite to be suspended in the electrolyte that can be removed by filtration. The cathode (where hydroxide forms) can be made from unalloyed titanium, graphite, or a more easily oxidized metal such as stainless steel or nickel.
Laboratory procedure
Electrolysis can be done with beakers, one containing a brine solution (salt water) and one containing pure water connected by a salt bridge. Anodes are made ideally from platinum group metals, which resist corrosion. Since corrosion is less severe at the cathode, it can be stainless steel or silver.
Manufacturer associations
The interests of chloralkali product manufacturers are represented at regional, national and international levels by associations such as Euro Chlor and The World Chlorine Council.
See also
| Wikimedia Commons has media related to Chloralkali process. |
References
- Fengmin Du, David M Warsinger, Tamanna I Urmi, Gregory P Thiel, Amit Kumar, John H Lienhard (2018). "Sodium hydroxide production from seawater desalination brine: process design and energy efficiency". Environmental Science & Technology. 52: 5949–5958. doi:10.1021/acs.est.8b01195. hdl:1721.1/123096. PMID 29669210.CS1 maint: multiple names: authors list (link)
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- R. Norris Shreve; Joseph Brink (1977). Chemical Process Industries (4th ed.). p. 219. ASIN B000OFVCCG.
- Tilley, R.J.D. (2004). Understanding solids: the science of materials. Understanding Solids: The Science of Materials. John Wiley and Sons. pp. 281–. Bibcode:2004usts.book.....T. ISBN 978-0-470-85276-7. Retrieved 22 October 2011.
- Thompson, M. de Kay (1911). Applied Electrochemistry. The MacMillan Company. pp. 89-90.
- Faraday, Michael (1849). Experimental Researches In Electricity. 1. London: The University of London.
- Landolt, D.; Ibl, N. (1972). "Anodic chlorate formation on platinized titanium". Journal of Applied Electrochemistry. Chapman and Hall Ltd. 2 (3): 201–210. doi:10.1007/BF02354977.CS1 maint: multiple names: authors list (link)
- Munichandraiah, N.; Sathyanarayana, S. (1988). "Insoluble anode of α-lead dioxide coated on titanium for electrosynthesis of sodium perchlorate". Journal of Applied Electrochemistry. Chapman and Hall Ltd. 18 (2): 314–316. doi:10.1007/BF01009281.CS1 maint: multiple names: authors list (link)
- Dinan, Charles (1927-10-15). The Corrosion of Durion Anodes (BSc). Massachusetts Institute of Technology. p. 4. Retrieved 2019-09-25.
- Hale, Arthur (1918). The Applications of Electrolysis in Chemical Industry. Longmans, Green, and Co. p. 13. Retrieved 2019-09-15.
- Denso, P. (1902). "Untersuchungen Über die Widerstandsfähigkeit von Platiniridium‐Anoden bei der Alkalichlorid‐Elektrolyse". Zeitschrift für Elektrochemie. Wilhelm Knapp. 8 (10): 149.
Further reading
- Bommaraju, Tilak V.; Orosz, Paul J.; Sokol, Elizabeth A.(2007). "Brine Electrolysis." Electrochemistry Encyclopedia. Cleveland: Case Western Rsserve University.