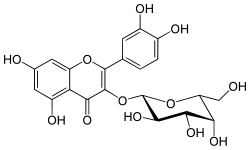
Hyperoside
Hyperoside is a chemical compound. It is the 3-O-galactoside of quercetin.
 | |
| Names | |
|---|---|
| IUPAC name
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl β-D-galactopyranoside | |
| Other names
Hyperozide Hyperasid Hyperosid Hyperin quercetin galactoside Quercetin-3-galactoside Quercetin-3-O-galactoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.892 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C21H20O12 | |
| Molar mass | 464.379 g·mol−1 |
| Density | 1.879 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Natural occurrences
Hyperoside has been isolated from Drosera rotundifolia, from the Lamiaceae Stachys sp. and Prunella vulgaris, from Rumex acetosella, Cuscuta chinensis seeds, from St John's wort and from Camptotheca acuminata.[1] It is one of the phenolic compounds in the invasive plant Carpobrotus edulis and contributes to the antibacterial[2] properties of the plant.
In Rheum nobile and R. rhaponticum, it serves as a UV blocker found in the bracts.
It is also found in Geranium niveum[3] and Taxillus kaempferi.[4]
gollark: Good (well, any) AR still costs a lot, but presumably costs will drop over time.
gollark: It's not like (bad) VR headsets are that costly compared to, say, (bad) laptops and such.
gollark: What?
gollark: I see.
gollark: Don't they require you to use a Facebook account?
References
- Li, Shiyou; Zhang, Zhizhen; Cain, Abigail; Wang, Bo; Long, Melissa; Taylor, Josephine (2005). "Antifungal Activity of Camptothecin, Trifolin, and Hyperoside Isolated fromCamptotheca acuminata". Journal of Agricultural and Food Chemistry. 53 (1): 32–7. doi:10.1021/jf0484780. PMID 15631505.
- Van Der Watt, Elmarie; Pretorius, Johan C (2001). "Purification and identification of active antibacterial components in Carpobrotus edulis L". Journal of Ethnopharmacology. 76 (1): 87–91. doi:10.1016/S0378-8741(01)00197-0. PMID 11378287.
- Calzada, F; Cerda-García-Rojas, CM; Meckes, M; Cedillo-Rivera, R; Bye, R; Mata, R (1999). "Geranins a and B, new antiprotozoal A-type proanthocyanidins from Geranium niveum". Journal of Natural Products. 62 (5): 705–9. doi:10.1021/np980467b. PMID 10346950.
- The constituents of Taxillus kaempferi and the host, Pinus thunbergii. I. Catechins and flavones of Taxillus kaempferi. Konishi T, Nishio T, Kiyosawa S, Fujiwara Y and Konoshima T, Yakugaku Zasshi., February 1996, volume 116, issue 2, pages 148-157 (article in Japanese)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.