Jaceidin
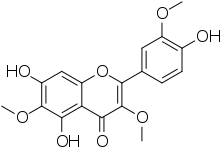
Jaceidin is an O-methylated flavonol. It can be found in Chamomilla recutita,[1] in Centaurea jacea and can be synthetized.[2] Jaceidin has many different characteristics, such as a molar mass of 360.31 g/mol. It also has a melting point of 130-135 °C.[3]
 | |
| Names | |
|---|---|
| IUPAC name
5,7-Dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,6-dimethoxychromen-4-one | |
| Other names
Jaceidine Quercetagetin 3,3',6-trimethyl ether | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H16O8 | |
| Molar mass | 360.318 g·mol−1 |
| Melting point | 130–135 °C (266–275 °F; 403–408 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Glycosides
- Jacein
gollark: Most fonts *don't*.
gollark: This is a very excellent RFC.
gollark: > The details for expanding the Unicode code point space are not covered in this document. Such details need to be worked out between the IETF, ISO, the Unicode Consortium, and various gods
gollark: We use bees as semi-"avian" carriers for some internal networking.
gollark: > Internationalizing IPv6 Using 128-Bit Unicodeoh BEES
References
- Repčák, Miroslav; Švehlı́Ková, Vanda; Imrich, Ján; Pihlaja, Kalevi (1999). "Jaceidin and chrysosplenetin chemotypes of Chamomilla recutita (L.) Rauschert". Biochemical Systematics and Ecology. 27 (7): 727–732. doi:10.1016/S0305-1978(98)00124-0.
- Fukui, K.; Matsumoto, T.; Nakamura, S.; Nakayama, M.; Horie, T. (1968). "The synthesis of jaceidin". Experientia. 24 (2): 108–109. doi:10.1007/BF02146923.
- "Jaceidin". Human Metabolome Database. HMDB0033819.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.