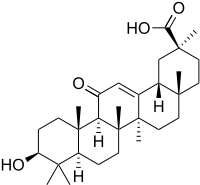
Enoxolone
Enoxolone (INN, BAN; also known as glycyrrhetinic acid or glycyrrhetic acid) is a pentacyclic triterpenoid derivative of the beta-amyrin type obtained from the hydrolysis of glycyrrhizic acid, which was obtained from the herb liquorice. It is used in flavoring and it masks the bitter taste of drugs like aloe and quinine. It is effective in the treatment of peptic ulcer and also has expectorant (antitussive) properties.[1] It has some additional pharmacological properties with possible antiviral, antifungal, antiprotozoal, and antibacterial activities.[2][3][4][5]
 | |
| Clinical data | |
|---|---|
| Trade names | Arthrodont, PruClair |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.769 |
| Chemical and physical data | |
| Formula | C30H46O4 |
| Molar mass | 470.6838 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Mechanism of action
Glycyrrhetinic acid inhibits the enzymes (15-hydroxyprostaglandin dehydrogenase and delta-13-prostaglandin) that metabolize the prostaglandins PGE-2 and PGF-2α to their respective 15-keto-13,14-dihydro metabolites which are inactive. This causes an increased level of prostaglandins in the digestive system. Prostaglandins inhibit gastric secretion but stimulate pancreatic secretion and mucous secretion in the intestines and markedly increase intestinal motility They also cause cell proliferation in the stomach. The effect on gastric acid secretion, promotion of mucous secretion and cell proliferation shows why licorice has potential in treating peptic ulcers.[6]
PGF-2α stimulates activity of the uterus during pregnancy and can cause abortion, therefore, licorice should not be taken during pregnancy.
The structure of glycyrrhetinic acid is similar to that of cortisone. Both molecules are flat and similar at position 3 and 11. This might be the basis for licorice's anti-inflammatory action.
3-β-D-(Monoglucuronyl)-18-β-glycyrrhetinic acid, a metabolite of glycyrrhetinic acid, inhibits the conversion of 'active' cortisol to 'inactive' cortisone in the kidneys.[7] This occurs via inhibition of the enzyme 11-β-hydroxysteroid dehydrogenase. As a result, cortisol levels are high within the collecting duct of the kidney. Cortisol has intrinsic mineralocorticoid properties (that is, it acts like aldosterone and increases sodium reabsorption) that work on ENaC channels in the collecting duct. Hypertension develops due to this mechanism of sodium retention. People often have high blood pressure with a low renin and low aldosterone blood level. The increased amounts of cortisol binds to the unprotected, unspecific mineralocorticoid receptors and induce sodium and fluid retention, hypokalaemia, high blood pressure and inhibition of the renin-angiotensin-aldosterone system. Therefore, licorice should not be given to patients with a known history of hypertension in doses sufficient to inhibit 11-β-hydroxysteroid dehydrogenase.[8]
Derivatives
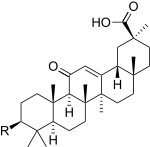
In glycyrrhetinic acid, the functional group (R) is a hydroxyl group. Research in 2005 demonstrated that with a proper functional group a very effective glycyrrhetinic artificial sweetener can be obtained.[9] When R is an anionic NHCO(CH2)CO2K side chain, the sweetening effect is found to 1200 times that of sugar (human sensory panel data). A shorter or longer spacer reduces the sweetening effect. One explanation is that the taste bud cell receptor has 1.3 nanometers (13 angstroms) available for docking with the sweetener molecule. In addition the sweetener molecule requires three proton donor positions of which two reside at the extremities to be able to interact efficiently with the receptor cavity.
A synthetic analog, carbenoxolone, was developed in Britain. Both glycyrrhetinic acid and carbenoxolone have a modulatory effect on neural signaling through gap junction channels.
Acetoxolone, the acetyl derivative of glycyrrhetinic acid, is a drug used in the treatment of peptic ulcer and gastroesophageal reflux disease.
See also
References
- Chandler RF (1985). "Liquorice, more than just a flavour". Canadian Pharmaceutical Journal (118): 420–4.
- Badam L (June 1997). "In vitro antiviral activity of indigenous glycyrrhizin, licorice and glycyrrhizic acid (Sigma) on Japanese encephalitis virus". The Journal of Communicable Diseases. 29 (2): 91–9. PMID 9282507.
- Fuji HY, Tian J, Luka C (1986). "Effect of glycyrrhetinic acid on influenza virus and pathogenic bacteria". Bull. Chin. Mater. Med. 11: 238–241.
- Guo N (October 1991). "[Protective effect of glycyrrhizine in mice with systemic Candida albicans infection and its mechanism]". Zhongguo Yi Xue Ke Xue Yuan Xue Bao. Acta Academiae Medicinae Sinicae. 13 (5): 380–3. PMID 1839259.
- Salari MH, Sohrabi N, Kadkhoda Z, Khalili MB (2003). "Antibacterial effects of Enoxolone on periodontopathogenic capnophilic bacteria isolated from specimens of periodontitis patients". Iranian Biomedical Journal. 7: 39–42.
- Baker, Michael E. (February 1994). "Licorice and enzymes other than 11β-hydroxysteroid dehydrogenase: An evolutionary perspective". Steroids. 59 (2): 136–141. doi:10.1016/0039-128X(94)90091-4.
|access-date=requires|url=(help) - Kato H, Kanaoka M, Yano S, Kobayashi M (June 1995). "3-Monoglucuronyl-glycyrrhetinic acid is a major metabolite that causes licorice-induced pseudoaldosteronism". The Journal of Clinical Endocrinology and Metabolism. 80 (6): 1929–33. doi:10.1210/jcem.80.6.7775643. PMID 7775643.
- van Uum SH (April 2005). "Liquorice and hypertension". The Netherlands Journal of Medicine. 63 (4): 119–20. PMID 15869038.
- Ijichi S, Tamagaki S (2005). "Molecular Design of Sweet Tasting Compounds Based on 3β-Amino-3β-deoxy-18β-glycyrrhetinic Acid: Amido Functionality Eliciting Tremendous Sweetness". Chemistry Letters. 34 (3): 356–357. doi:10.1246/cl.2005.356. Retrieved 2010-09-28.
Further reading
- Saponin Glycosides, by Georges-Louis Friedli, URL accessed Sept 2010.