Aroma compound
An aroma compound, also known as an odorant, aroma, fragrance, or flavor, is a chemical compound that has a smell or odor. For a chemical compound to have a smell or odor it must be sufficiently volatile to be transported to the olfactory system in the upper part of the nose.

Generally molecules meeting this specification have molecular weights of less than 300. Flavors affect both the sense of taste and smell, whereas fragrances affect only smell. Flavors tend to be naturally occurring, and fragrances tend to be synthetic.[1]
Aroma compounds can be found in food, wine, spices, floral scent, perfumes, fragrance oils, and essential oils. For example, many form biochemically during the ripening of fruits and other crops. In wines, most form as byproducts of fermentation. Also, many of the aroma compounds play a significant role in the production of flavorants, which are used in the food service industry to flavor, improve, and generally increase the appeal of their products.
An odorizer may add a detectable odor to a dangerous odorless substance, like propane, natural gas, or hydrogen, as a safety measure.
Aroma compounds classified by structure
Esters
| Compound name | Fragrance | Natural occurrence | Chemical structure |
|---|---|---|---|
| Geranyl acetate | Fruity, Floral |
Rose | |
| Methyl formate | Ethereal | ||
| Methyl acetate | Sweet, nail polish Solvent |
||
| Methyl propionate Methyl propanoate |
Sweet, fruity, rum-like | ||
| Methyl butyrate Methyl butanoate |
Fruity | Apple Pineapple |
|
| Ethyl acetate | Sweet, solvent | Wine | |
| Ethyl butyrate Ethyl butanoate |
Fruity | Orange, Pineapple | |
| Isoamyl acetate | Fruity, Banana Pear |
Banana plant | |
| Pentyl butyrate Pentyl butanoate |
Fruity | Pear Apricot |
|
| Pentyl pentanoate | Fruity | Apple | |
| Octyl acetate | Fruity | Orange | |
| Benzyl acetate | Fruity, Strawberry | Strawberries | |
| Methyl anthranilate | Fruity | Grape | 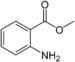 |
| Hexyl acetate | Floral, Fruity | Apple, Plum |  |
Linear terpenes
| Compound name | Fragrance | Natural occurrence | Chemical structure |
|---|---|---|---|
| Myrcene | Woody, complex | Verbena, Bay leaf | |
| Geraniol | Rose, flowery | Geranium, Lemon | |
| Nerol | Sweet rose, flowery | Neroli, Lemongrass | 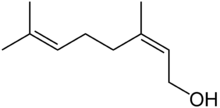 |
| Citral, lemonal Geranial, neral |
Lemon | Lemon myrtle, Lemongrass | |
| Citronellal | Lemon | Lemongrass | |
| Citronellol | Lemon | Lemongrass, rose Pelargonium |
|
| Linalool | Floral, sweet Woody |
Coriander, Sweet basil, Lavender, Honeysuckle | |
| Nerolidol | Woody, fresh bark | Neroli, ginger Jasmine |
|
| Ocimene | Fruity, Floral | Mango, Curcuma amada |
Cyclic terpenes
| Compound name | Fragrance | Natural occurrence | Chemical structure |
|---|---|---|---|
| Limonene | Orange | Orange, lemon | |
| Camphor | Camphor | Camphor laurel | |
| Menthol | Menthol | Mentha | |
| Carvone1 | Caraway or Spearmint | Caraway, dill, spearmint |
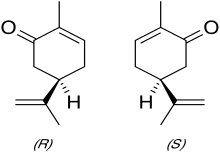 |
| Terpineol | Lilac | Lilac, cajuput | |
| alpha-Ionone | Violet, woody | Violet | 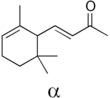 |
| Thujone | Minty | Wormwood, lilac, juniper |
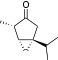 |
| Eucalyptol | Eucalyptus | Eucalyptus | 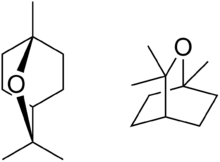 |
| Jasmone | spicy, fruity, floral in dilution | Jasmine, Honeysuckle |  |
Note: Carvone, depending on its chirality, offers two different smells.
Aromatic
| Compound name | Fragrance | Natural occurrence | Chemical structure |
|---|---|---|---|
| Benzaldehyde | Almond | Bitter almond | |
| Eugenol | Clove | Clove | 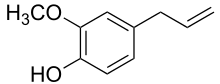 |
| Cinnamaldehyde | Cinnamon | Cassia Cinnamon |
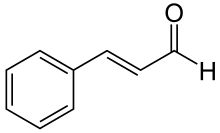 |
| Ethyl maltol | Cooked fruit Caramelized sugar |
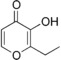 | |
| Vanillin | Vanilla | Vanilla | 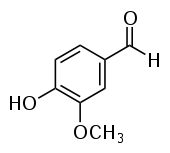 |
| Anisole | Anise | Anise | |
| Anethole | Anise | Anise Sweet basil |
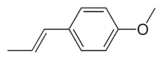 |
| Estragole | Tarragon | Tarragon | 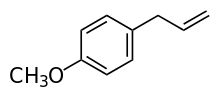 |
| Thymol | Thyme | Thyme |
Amines
| Compound name | Fragrance | Natural occurrence | Chemical structure |
|---|---|---|---|
| Trimethylamine | Fishy Ammonia |
||
| Putrescine Diaminobutane |
Rotting flesh | Rotting flesh | |
| Cadaverine | Rotting flesh | Rotting flesh | |
| Pyridine | Fishy | Belladonna | |
| Indole | Fecal Flowery |
Feces Jasmine |
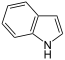 |
| Skatole | Fecal | Feces (diluted) Orange Blossoms |
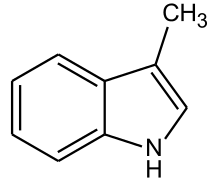 |
Other aroma compounds
Alcohols
- Furaneol (strawberry)
- 1-Hexanol (herbaceous, woody)
- cis-3-Hexen-1-ol (fresh cut grass)
- Menthol (peppermint)
Aldehydes
High concentrations of aldehydes tend to be very pungent and overwhelming, but low concentrations can evoke a wide range of aromas.
- Acetaldehyde (ethereal)
- Hexanal (green, grassy)
- cis-3-Hexenal (green tomatoes)
- Furfural (burnt oats)
- Hexyl cinnamaldehyde
- Isovaleraldehyde – nutty, fruity, cocoa-like
- Anisic aldehyde – floral, sweet, hawthorn. It is a crucial component of chocolate, vanilla, strawberry, raspberry, apricot, and others.
- Cuminaldehyde (4-propan-2-ylbenzaldehyde) – Spicy, cumin-like, green
Esters
- Fructone (fruity, apple-like)
- Ethyl methylphenylglycidate (Strawberry)
- alpha-Methylbenzyl acetate (Gardenia)
Ketones
- Cyclopentadecanone (musk-ketone)[2]
- Dihydrojasmone (fruity woody floral)
- Oct-1-en-3-one (blood, metallic, mushroom-like)[3]
- 2-Acetyl-1-pyrroline (fresh bread, jasmine rice)
- 6-Acetyl-2,3,4,5-tetrahydropyridine (fresh bread, tortillas, popcorn)
Lactones
- gamma-Decalactone intense peach flavor
- gamma-Nonalactone coconut odor, popular in suntan lotions
- delta-Octalactone creamy note
- Jasmine lactone powerful fatty-fruity peach and apricot
- Massoia lactone powerful creamy coconut
- Wine lactone sweet coconut odor
- Sotolon (maple syrup, curry, fenugreek)
Thiols
- Thioacetone (2-propanethione) A lightly studied organosulfur. Its smell is so potent it can be detected several hundred meters downwind mere seconds after a container is opened.
- Allyl thiol (2-propenethiol; allyl mercaptan; CH2=CHCH2SH) (garlic volatiles and garlic breath)[4]
- (Methylthio)methanethiol (CH3SCH2SH), the "mouse thiol", found in mouse urine and functions as a semiochemical for female mice[5]
- Ethanethiol, commonly called ethyl mercaptan (added to propane or other liquefied-petroleum gases used as fuel gases)
- 2-Methyl-2-propanethiol, commonly called tert-butyl mercaptan, is added as a blend of other components to natural gas used as fuel gas.
- Butane-1-thiol, commonly called butyl mercaptan, is a chemical intermediate.
- Grapefruit mercaptan (grapefruit)
- Methanethiol, commonly called methyl mercaptan (after eating Asparagus)
- Furan-2-ylmethanethiol, also called furfuryl mercaptan (roasted coffee)
- Benzyl mercaptan (leek or garlic-like)
Miscellaneous compounds
- Methylphosphine and dimethylphosphine (garlic-metallic, two of the most potent odorants known)[3]
- Phosphine (zinc phosphide poisoned bait)
- Diacetyl (butter flavor)
- Acetoin (butter flavor)
- Nerolin (orange flowers)
- Tetrahydrothiophene (added to natural gas)
- 2,4,6-Trichloroanisole (cork taint)
- Substituted pyrazines
Aroma-compound receptors
Animals that are capable of smell detect aroma compounds with their olfactory receptors. Olfactory receptors are cell-membrane receptors on the surface of sensory neurons in the olfactory system that detect airborne aroma compounds. Aroma compounds can then be identified by Gas Chromatography-Olfactometry (GC-O), which involves a human operator sniffing the GC effluent.[6]
In mammals, olfactory receptors are expressed on the surface of the olfactory epithelium in the nasal cavity.
Safety and regulation
In 2005–06, fragrance mix was the third-most-prevalent allergen in patch tests (11.5%).[7] 'Fragrance' was voted Allergen of the Year in 2007 by the American Contact Dermatitis Society. A recent academic study in the United States has shown that "34.7 % of the population reported health problems, such as migraine headaches and respiratory difficulties, when exposed to fragranced products".[8]
The composition of fragrances is usually not disclosed in the label of products, hiding the actual chemicals of the formula, which raises concerns among some consumers.[9] In the United States, this is because the law regulating cosmetics protects trade secrets.[10]
In the United States, fragrances are regulated by the Food and Drug Administration if present in cosmetics or drugs, by the Consumer Products Safety Commission if present in consumer products.[10] No pre-market approval is required, except for drugs. Fragrances are also generally regulated by the Toxic Substances Control Act of 1976 that "grandfathered" existing chemicals without further review or testing and put the burden of proof that a new substance is not safe on the EPA. The EPA, however, does not conduct independent safety testing but relies on data provided by the manufacturer.[11]
A 2019 study of the top-selling skin moisturizers from amazon.com, Target, and Walmart found 45% of those marketed as "fragrance-free" contained fragrance.[12]
List of chemicals used as fragrances
In 2010 the International Fragrance Association published a list of 3,059 chemicals used in 2011 based on a voluntary survey of its members. It was estimated to represent about 90% of the world's production volume of fragrances.[13]
See also
- Flavour and Fragrance Journal
- Fragrances of the World
- Foodpairing
- Odor
- Odor detection threshold
- Olfaction
- Olfactory system
- Olfactory receptor
- Odorizer, a device for adding an odorant to gas flowing through a pipe
- Pheromone
- Aroma of wine
- Eau de toilette
References
- Fahlbusch, Karl-Georg; Hammerschmidt, Franz-Josef; Panten, Johannes; Pickenhagen, Wilhelm; Schatkowski, Dietmar; Bauer, Kurt; Garbe, Dorothea; Surburg, Horst. "Flavors and Fragrances". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_141.
- Gane, S; Georganakis, D; Maniati, K; Vamvakias, M; Ragoussis, N; Skoulakis, EMC; Turin, L (2013). "Molecular-vibration-sensing component in human olfaction". PLOS ONE. 8 (1): e55780. Bibcode:2013PLoSO...855780G. doi:10.1371/journal.pone.0055780. PMC 3555824. PMID 23372854.
- Glindemann, D.; Dietrich, A.; Staerk, H.; Kuschk, P. (2005). "The Two Odors of Iron when Touched or Pickled: (Skin) Carbonyl Compounds and Organophosphines". Angewandte Chemie International Edition. 45 (42): 7006–7009. doi:10.1002/anie.200602100. PMID 17009284.
- Block, E. (2010). Garlic and Other Alliums: The Lore and the Science. Royal Society of Chemistry. ISBN 978-0-85404-190-9.
- Lin, D.Y.; Zhang, S.Z.; Block, E.; Katz, L.C. (2005). "Encoding social signals in the mouse main olfactory bulb". Nature. 434 (7032): 470–477. Bibcode:2005Natur.434..470L. doi:10.1038/nature03414. PMID 15724148. S2CID 162036.
- Brattoli, M; Cisternino, E; Dambruoso, PR; de Gennaro, G; Giungato, P; Mazzone, A; Palmisani, J; Tutino, M (5 December 2013). "Gas chromatography analysis with olfactometric detection (GC-O) as a useful methodology for chemical characterization of odorous compounds". Sensors (Basel, Switzerland). 13 (12): 16759–800. doi:10.3390/s131216759. PMC 3892869. PMID 24316571.
- Zug KA, Warshaw EM, Fowler JF Jr, Maibach HI, Belsito DL, Pratt MD, Sasseville D, Storrs FJ, Taylor JS, Mathias CG, Deleo VA, Rietschel RL, Marks J. Patch-test results of the North American Contact Dermatitis Group 2005–2006. Dermatitis. 2009 May–Jun;20(3):149-60.
- Anne Steinemann, "Fragranced consumer products: exposures and effects from emissions", Air Quality, Atmosphere & Health, December 2016, Volume 9, Issue 8, pp 861–866.
- Anne C. Steinemann et al., "Fragranced Consumer Products: Chemicals Emitted, Ingredients Unlisted", Environmental Impact Assessment Review, Vol. 31, Issue 3, April 2011, pp. 328-333.
- Fragrances in Cosmetics
- Randall Fitzgerald (2006). The Hundred Year Lie. Dutton, 2006. p. 23. ISBN 978-0-525-94951-0.
- 'Hypoallergenic' And 'Fragrance-Free' Moisturizer Claims Are Often False
- "IFRA Survey:Transparency List". IFRA. Retrieved December 3, 2014.
