Furan-2-ylmethanethiol
Furan-2-ylmethanethiol is an organic compound containing a furan substituted with a sulfanylmethyl group. It is a clear colourless liquid when pure, but it becomes yellow coloured upon prolonged standing. It possesses a strong odour of roasted coffee and a bitter taste. It is a key component of the aroma of roasted coffee.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(Furan-2-yl)methanethiol | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| 383594 | |
| ChemSpider | |
| ECHA InfoCard | 100.002.390 |
| EC Number |
|
| MeSH | furfuryl+mercaptan |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 3336 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H6OS | |
| Molar mass | 114.16 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Roasted coffee, Caramel, Sulfurous, Waxy |
| Density | 1.132 g cm−3 |
| Boiling point | 155 °C; 311 °F; 428 K |
| Vapor pressure | 531 Pa |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H226 |
| Flash point | 45 °C (113 °F; 318 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
100-200 mg kg−1 (mouse) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Furan-2-ylmethanol is easily prepared by reacting furfuryl alcohol with thiourea in hydrochloric acid via an intermediate isothiouronium salt which is hydrolized to the thiol by heating with sodium hydroxide.[1]
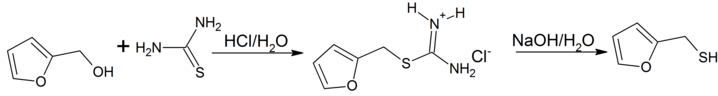
Synthesis of furfuryl mercaptane (Furan-2-ylmethanethiol)
gollark: No it doesn't.
gollark: There will probably always be scarce things.
gollark: You do realise, though, that even with free *material goods*, you do still need, say, spaceship designers? Material scarcity and general scarcity are separate.
gollark: Yes it does.
gollark: Some episodes later... "We need X spare part for the spaceship!"
References
- Preparation of furfuryl mercaptane http://www.orgsyn.org/demo.aspx?prep=CV4P0491
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
