Isomalt
Isomalt is a sugar substitute, a type of sugar alcohol used primarily for its sugar-like physical properties. It has little to no impact on blood sugar levels, and does not stimulate the release of insulin.[1] It also does not promote tooth decay, that is, it is tooth-friendly. Its energy value is 2 kcal per gram, half that of sugars.[2] However, like most sugar alcohols, it carries a risk of gastric distress, including flatulence and diarrhea, when consumed in large quantities (above about 20–30 g (1 oz) per day).[1] Isomalt may prove upsetting to the intestinal tract because it is incompletely absorbed in the small intestine, and when polyols pass into the large intestine, they can cause osmotically induced diarrhea[3] and stimulate the gut flora, causing flatulence.[1] As with dietary fibers, regular consumption of isomalt can lead to desensitisation, decreasing the risk of intestinal upset.[1] Isomalt can be blended with high-intensity sweeteners such as sucralose, giving a mixture that has the same sweetness as sugar.
 | |
| Names | |
|---|---|
| IUPAC name
(2R,3R,4R,5R)-6-[[(2S,3R,4S,5S,6R)- 3,4,5-Trihydroxy-6-(hydroxymethyl)-2-tetrahydropyranyl]oxy]hexane-1,2,3,4,5-pentol | |
| Other names
1-O-alpha-D-Glucopyranosyl-D-mannitol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.040.096 |
| E number | E953 (glazing agents, ...) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H24O11 | |
| Molar mass | 344.313 g·mol−1 |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
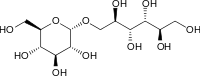
Isomalt is an equimolar mixture of two mutually diastereomeric disaccharides, each composed of two sugars: glucose and mannitol (α-D-glucopyranosido-1,6-mannitol) and also glucose and sorbitol (α-D-glucopyranosido-1,6-sorbitol). Complete hydrolysis of isomalt yields glucose (50%), sorbitol (25%), and mannitol (25%).[4] It is an odorless, white, crystalline substance containing about 5% water of crystallisation. Isomalt has a minimal cooling effect (positive heat of solution[5]), lower than many other sugar alcohols, in particular, xylitol and erythritol.
Isomalt is manufactured in a two-stage process in which sucrose is first transformed into isomaltulose, a reducing disaccharide (6-O-α-D-glucopyranosido-D-fructose). The isomaltulose is then hydrogenated, using a Raney nickel catalyst. The final product — isomalt — is an equimolar composition of 6-O-α-D-glucopyranosido-D-sorbitol (1,6-GPS) and 1-O-α-D-glucopyranosido-D-mannitol-dihydrate (1,1-GPM-dihydrate).
Isomalt has been approved for use in the United States since 1990. It is also permitted for use in Australia, New Zealand, Canada, Mexico, Iran, the European Union, and other countries.
Uses
Isomalt is widely used for the production of sugar-free candy, especially hard-boiled candy, because it resists crystallization much better than the standard combinations of sucrose and corn syrup. It is used in sugar sculpture for the same reason.[6]
References
- "604. Isomalt (WHO Food Additives Series 20)". www.inchem.org. Retrieved 2017-09-28.
- "Position of The American Dietetic Association (use of nutritive and nonnutritive sweeteners)". J. Am. Diet. Assoc. 98: 580–587. 1998.
- Grenby, Trevor H. (2012-12-06). Advances in Sweeteners. Springer Science & Business Media. ISBN 9781461312291.
- Joint Expert Committee on Food Additives (JECFA). "Isomalt". International Programme on Chemical Safety (IPCS). Retrieved 15 December 2012.
- Wohlfarth, Christian. CRC Handbook of Enthalpy Data of Polymer-Solvent Systems. CRC Press, 2006. Google Books result: ISBN 0-8493-9361-2
- The Oxford Companion to Sugar and Sweets. Oxford University Press. 2015-04-01. ISBN 9780199313624.
External links

- IPCS information page on Isomalt
