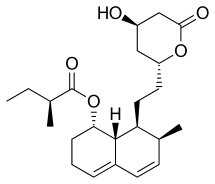
Mevastatin
Mevastatin (compactin, ML-236B) is a hypolipidemic agent that belongs to the statins class.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.131.541 |
| Chemical and physical data | |
| Formula | C23H34O5 |
| Molar mass | 390.520 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
It was isolated from the mold Penicillium citrinum by Akira Endo in the 1970s, and he identified it as a HMG-CoA reductase inhibitor,[1] i.e., a statin. Mevastatin might be considered the first statin drug;[2] clinical trials on mevastatin were performed in the late 1970s in Japan, but it was never marketed.[3] The first statin drug available to the general public was lovastatin.
Mevastatin has since been derivatized to the compound pravastatin, which is a pharmaceutical used in the lowering of cholesterol and preventing cardiovascular disease.
In vitro, it has antiproliferative properties.[4]
A British group isolated the same compound from Penicillium brevicompactum, named it compactin, and published their results in 1976.[5] The British group mentions antifungal properties with no mention of HMG-CoA reductase inhibition.
High doses inhibit growth and proliferation of melanoma cells.[6]
Biosynthesis
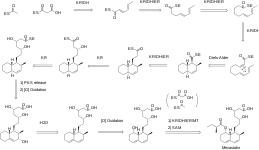
Biosynthesis of mevastatin is primarily accomplished via a type 1 PKS pathway it proceeds in the PKS pathway as seen in figure 1 until it reaches a hexaketide state where it undergoes a Diels-Alder cyclization. After cyclization it continues via the PKS pathway to a nonaketide after which it is released and undergoes oxidation and dehydration. It is presumed that the oxidations are preformed by a polypeptide that is similar to cytochrome p450 monooxygenase, which is encoded by mlcC within the mevastatin gene. Lastly the biosynthesis is completed by the PKS facilitating the addition of a diketide sidechain and a methylation by SAM.[7] Figure 1 shows mevastatin in its acid form but it can also be in the more commonly seen lactone form. This pathway was first observed in Penicillium cilrinum and was later discovered that another type of fungus, Penicillium brevicompaetum also produced mevastatin via a PKS pathway.
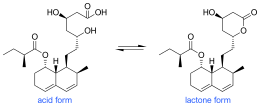
Pharmacology
Sustained elevations of cholesterol in the blood increase the risk of cardiovascular disease. Mevastatin acts to lowers hepatic production of cholesterol by competitively inhibiting HMG-CoA reductase, the enzyme that catalyzes the rate-limiting step in the cholesterol biosynthesis pathway via the mevalonic acid pathway. When hepatic cholesterol levels are decreased it causes an increased uptake of low density lipoprotein (LDL) cholesterol and reduces cholesterol levels in the circulation.[8] It has also been shown that mevastatin upregulates endothelial nitric oxide synthase (eNOS), which is essential for maintaining a healthy cardiovascular system.[9]
See also
- Medicinal molds
References
- Endo A, Kuroda M, Tsujita Y (December 1976). "ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium". The Journal of Antibiotics. 29 (12): 1346–8. doi:10.7164/antibiotics.29.1346. PMID 1010803.
- "The story of statins". Archived from the original on December 21, 2008.
- Endo A (October 2004). "The origin of the statins. 2004". Atherosclerosis. Supplements. 5 (3): 125–30. doi:10.1016/j.atherosclerosissup.2004.08.033. PMID 15531285.
- Wächtershäuser A, Akoglu B, Stein J (July 2001). "HMG-CoA reductase inhibitor mevastatin enhances the growth inhibitory effect of butyrate in the colorectal carcinoma cell line Caco-2". Carcinogenesis. 22 (7): 1061–7. doi:10.1093/carcin/22.7.1061. PMID 11408350.
- Brown AG, Smale TC, King TJ, Hasenkamp R, Thompson RH (1976). "Crystal and molecular structure of compactin, a new antifungal metabolite from Penicillium brevicompactum". Journal of the Chemical Society. Perkin Transactions 1 (11): 1165–70. doi:10.1039/P19760001165. PMID 945291.
- Glynn SA, O'Sullivan D, Eustace AJ, Clynes M, O'Donovan N (January 2008). "The 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, simvastatin, lovastatin and mevastatin inhibit proliferation and invasion of melanoma cells". BMC Cancer. 8: 9. doi:10.1186/1471-2407-8-9. PMC 2253545. PMID 18199328.
- Abe Y, Suzuki T, Ono C, Iwamoto K, Hosobuchi M, Yoshikawa H (July 2002). "Molecular cloning and characterization of an ML-236B (compactin) biosynthetic gene cluster in Penicillium citrinum". Molecular Genetics and Genomics. 267 (5): 636–46. doi:10.1007/s00438-002-0697-y. PMID 12172803.
- "Mevastatin". PubChem. U.S. National Library of Medicine. Retrieved 2016-06-04.
- Amin-Hanjani S, Stagliano NE, Yamada M, Huang PL, Liao JK, Moskowitz MA (April 2001). "Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice". Stroke. 32 (4): 980–6. doi:10.1161/01.STR.32.4.980. PMID 11283400.