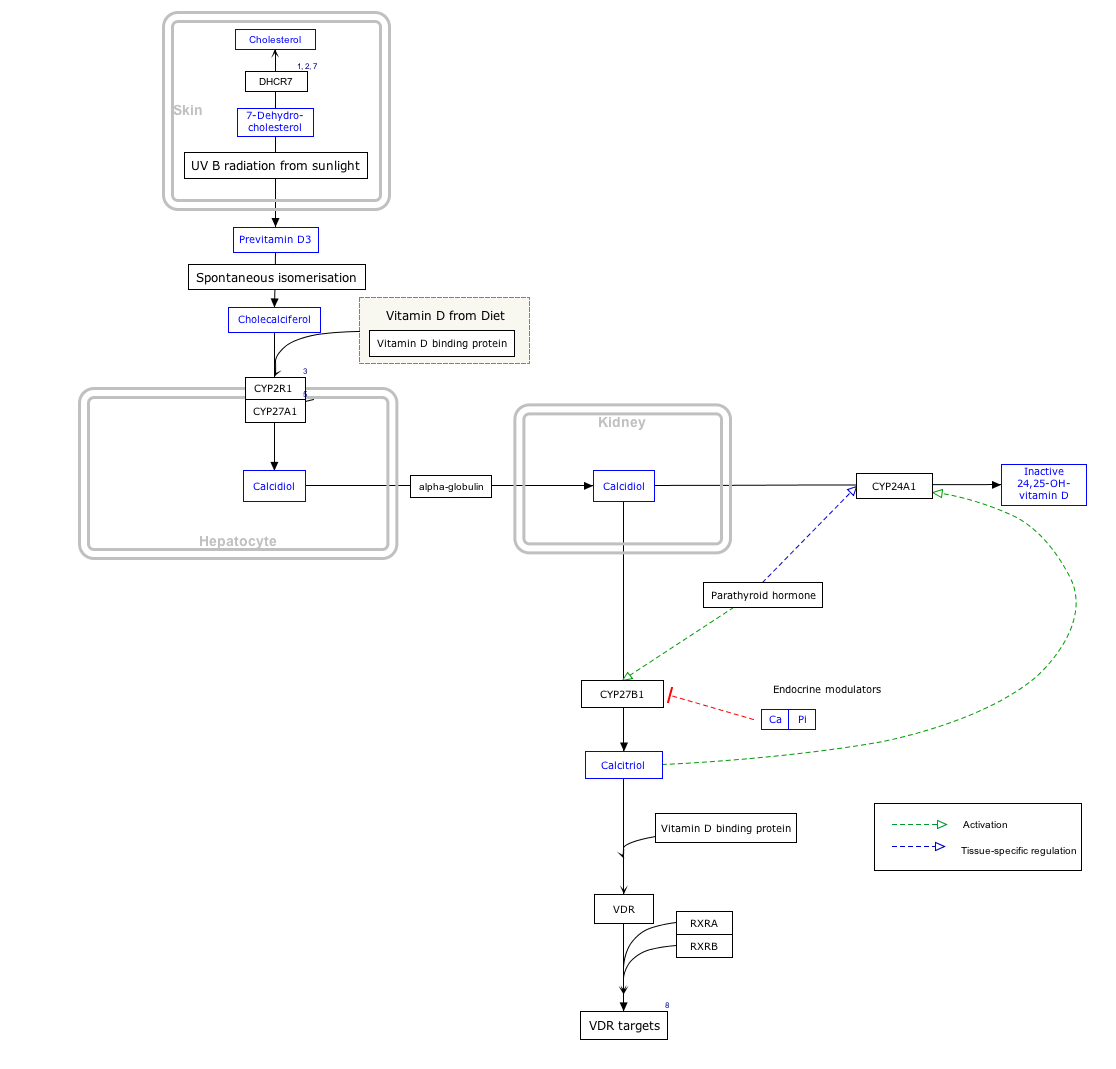
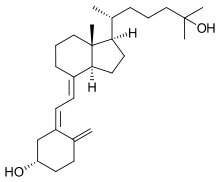
Calcifediol
Calcifediol, also known as calcidiol, 25-hydroxycholecalciferol, or 25-hydroxyvitamin D (abbreviated 25(OH)D),[1] is a prehormone that is produced in the liver by hydroxylation of vitamin D3 (cholecalciferol) by the enzyme cholecalciferol 25-hydroxylase. Physicians worldwide measure this metabolite to determine a patient's vitamin D status.[2][3] At a typical daily intake of vitamin D3, its full conversion to calcifediol takes approximately 7 days.[4]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(6R)-6-[(1R,3aR,4E,7aR)-4-[(2Z)-2-[(5S)-5- | |
| Other names
25-Hydroxyvitamin D3 25-Hydroxycholecalciferol Calcidiol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.039.067 |
| MeSH | Calcifediol |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H44O2 | |
| Molar mass | 400.64 g/mol |
| Pharmacology | |
| A11CC06 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Calcifediol is then converted in the kidneys (by the enzyme 25(OH)D-1α-hydroxylase) into calcitriol (1,25-(OH)2D3), a secosteroid hormone that is the active form of vitamin D. It can also be converted into 24-hydroxycalcidiol in the kidneys via 24-hydroxylation.[5][6]
Blood test
In medicine, a 25-hydroxy vitamin D (calcifediol) blood test is used to determine how much vitamin D is in the body.[7] The blood concentration of calcifediol is considered the best indicator of vitamin D status.[2]
This test can be used to diagnose vitamin D deficiency, and it is indicated in patients with high risk for vitamin D deficiency and when the results of the test would be used as supporting evidence for beginning aggressive therapies.[8] Patients with osteoporosis, chronic kidney disease, malabsorption, obesity, and some other infections may be high risk and thus have greater indication for this test.[8] Although vitamin D deficiency is common in some populations including those living at higher latitudes or with limited sun exposure, the 25(OH)D test is not indicated for entire populations.[8] Physicians may advise low risk patients to take over-the-counter vitamin D in place of having screening.[8]
It is the most sensitive measure,[9] though experts have called for improved standardization and reproducibility across different laboratories.[2] According to MedlinePlus, the normal range of calcifediol is 30.0 to 74.0 ng/mL.[7] The normal range varies widely depending on several factors, including age and geographic location. A broad reference range of 20–150 nmol/L (8-60 ng/mL) has also been suggested,[10] while other studies have defined levels below 80 nmol/L (32 ng/mL) as indicative of vitamin D deficiency.[11]
US labs generally report 25(OH)D levels as ng/mL. Other countries often use nmol/L. Multiply ng/mL by 2.5 to convert to nmol/L.
Clinical significance
Increasing calcifediol levels are associated with increasing fractional absorption of calcium from the gut up to levels of 80 nmol/L (32 ng/mL).[2] Urinary calcium excretion balances intestinal calcium absorption and does not increase with calcifediol levels up to ~400 nmol/L (160 ng/mL).[12]
A study by Cedric F. Garland and Frank C. Garland of the University of California, San Diego analyzed the blood from 25,000 volunteers from Washington County, Maryland, finding that those with the highest levels of calcifediol had a risk of colon cancer that was one-fifth of typical rates.[13] However, randomized controlled trials failed to find a significant correlation between low-dosage (400iu/day) vitamin D supplementation and the risk of colon cancer.[14]
A population study in Copenhagen, Denmark, found a correlation between both low and high serum levels and increased mortality, with a level of 50–60 nmol/L being associated with the lowest mortality. The study did not show causation.[15][16]
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- The interactive pathway map can be edited at WikiPathways: "VitaminDSynthesis_WP1531".
History
Research in the laboratory of Hector DeLuca identified 25(OH)D in the 1968 and showed that the liver was necessary for its formation.[17] The enzyme responsible for this, cholecalciferol 25-hydroxylase, was isolated by Michael F. Holick in 1972.[18]
See also
- Hypervitaminosis D
- Hypovitaminosis D
- Vitamin D
- Health effects of Vitamin D
References
- "Nomenclature of Vitamin D. Recommendations 1981. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) Archived 2017-08-23 at the Wayback Machine" reproduced at the Queen Mary, University of London website. Retrieved 21 March 2010.
- Heaney RP (December 2004). "Functional indices of vitamin D status and ramifications of vitamin D deficiency". The American Journal of Clinical Nutrition. 80 (6 Suppl): 1706S–9S. doi:10.1093/ajcn/80.6.1706S. PMID 15585791.
- Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP (April 2014). "Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials". BMJ. 348: g2035. doi:10.1136/bmj.g2035. PMC 3972415. PMID 24690624.
- Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW (June 2008). "25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions". The American Journal of Clinical Nutrition. 87 (6): 1738–42. doi:10.1093/ajcn/87.6.1738. PMID 18541563.
- Bender DA, Mayes PA (2006). "Micronutrients: Vitamins & Minerals". In Rodwell VW, Murray RF, Harper HW, Granner DK, Mayes PA (eds.). Harper's Illustrated Biochemistry. New York: Lange/McGraw-Hill. pp. 492–3. ISBN 978-0-07-146197-9. Retrieved December 10, 2008 through Google Book Search.
- Institute of Medicine (1997). "Vitamin D". Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, D.C: National Academy Press. p. 254. doi:10.17226/5776. ISBN 978-0-309-06403-3. PMID 23115811.
- "25-hydroxy vitamin D test: Medline Plus". Retrieved 21 March 2010.
- American Society for Clinical Pathology, "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American Society for Clinical Pathology, retrieved August 1, 2013, which cites
- Sattar N, Welsh P, Panarelli M, Forouhi NG (January 2012). "Increasing requests for vitamin D measurement: costly, confusing, and without credibility". Lancet. 379 (9811): 95–6. doi:10.1016/S0140-6736(11)61816-3. PMID 22243814.
- Bilinski KL, Boyages SC (July 2012). "The rising cost of vitamin D testing in Australia: time to establish guidelines for testing". The Medical Journal of Australia. 197 (2): 90. doi:10.5694/mja12.10561. PMID 22794049.
- Lu CM (May 2012). "Pathology consultation on vitamin D testing: clinical indications for 25(OH) vitamin D measurement". American Journal of Clinical Pathology. American Society for Clinical Pathology. 137 (5): 831–2. doi:10.1309/ajcp2gp0ghkqrcoe. PMID 22645788., which cites
- Arya SC, Agarwal N (May 2012). "The measurement of vitamin D₃requires maintaining quality control". American Journal of Clinical Pathology. 137 (5): 832, author reply 833. doi:10.1309/AJCP2GP0GHKQRCOE. PMID 22523224.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. (July 2011). "Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline". The Journal of Clinical Endocrinology and Metabolism. 96 (7): 1911–30. doi:10.1210/jc.2011-0385. PMID 21646368.
- Institute of Medicine (1997), p. 259
- Bender DA (2003). "Vitamin D". Nutritional biochemistry of the vitamins. Cambridge: Cambridge University Press. ISBN 978-0-521-80388-5. Retrieved December 10, 2008 through Google Book Search.
- Hollis BW (February 2005). "Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D". The Journal of Nutrition. 135 (2): 317–22. doi:10.1093/jn/135.2.317. PMID 15671234.
- Kimball SM, Ursell MR, O'Connor P, Vieth R (September 2007). "Safety of vitamin D3 in adults with multiple sclerosis". The American Journal of Clinical Nutrition. 86 (3): 645–51. doi:10.1093/ajcn/86.3.645. PMID 17823429.
- Maugh II, Thomas H. "Frank C. Garland dies at 60; epidemiologist helped show importance of vitamin D: Garland and his brother Cedric were the first to demonstrate that vitamin D deficiencies play a role in cancer and other diseases.", Los Angeles Times, August 31, 2010. Accessed September 4, 2010.
- Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, et al. (February 2006). "Calcium plus vitamin D supplementation and the risk of colorectal cancer". The New England Journal of Medicine. 354 (7): 684–96. doi:10.1056/NEJMoa055222. PMID 16481636.
- "Too much vitamin D can be as unhealthy as too little" (Press release). University of Copenhagen. May 29, 2012. Retrieved 2015-05-27.
- Durup D, Jørgensen HL, Christensen J, Schwarz P, Heegaard AM, Lind B (August 2012). "A reverse J-shaped association of all-cause mortality with serum 25-hydroxyvitamin D in general practice: the CopD study". The Journal of Clinical Endocrinology and Metabolism. Endocrine Society. 97 (8): 2644–52. doi:10.1210/jc.2012-1176. PMID 22573406.
- Ponchon G, Kennan AL, DeLuca HF (November 1969). ""Activation" of vitamin D by the liver". The Journal of Clinical Investigation. 48 (11): 2032–7. doi:10.1172/JCI106168. PMC 297455. PMID 4310770.
- Holick MF, DeLuca HF, Avioli LV (January 1972). "Isolation and identification of 25-hydroxycholecalciferol from human plasma". Archives of Internal Medicine. 129 (1): 56–61. doi:10.1001/archinte.1972.00320010060005. PMID 4332591.