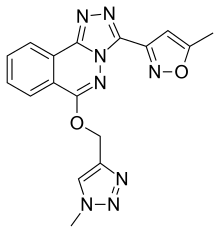
α5IA
α5IA (LS-193,268) is a nootropic drug invented in 2004 by a team working for Merck, Sharp and Dohme, which acts as a subtype-selective inverse agonist at the benzodiazepine binding site on the GABAA receptor. It binds to the α1, α2, α3 and α5 subtypes, but shows much higher efficacy at the α5 subtype, and acts either as a weak partial agonist or inverse agonist at the other subtypes, with its partial agonist effect at α2 likely to be responsible for the lack of anxiety produced by this drug when compared to older α5-preferring inverse agonists such as L-655,708.[1][2]
 | |
| Clinical data | |
|---|---|
| Other names | LS-193,268 |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H14N8O2 |
| Molar mass | 362.353 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
The α5 subtype is expressed predominantly in the hippocampus, an area of the brain involved with learning and memory, and activation of this subtype is thought to be largely responsible for producing the cognitive side effects displayed by many benzodiazepine and nonbenzodiazepine drugs, such as amnesia and difficulties with learning and memory. This led researchers to conclude that a drug acting as an inverse agonist at this subtype should have the opposite effect and enhance learning and memory.[3][4][5]
Older non-selective inverse agonists at the benzodiazepine site such as DMCM are associated with a range of other effects including anxiety and convulsions, but because α5IA acts specifically at the α5 subtype it produces nootropic effects in animal studies, yet without any significant anxiogenic or pro-convulsant effects. This gives α5IA the potential to be a useful drug either to be used alongside benzodiazepines to counteract their cognitive side effects, or by itself as a nootropic with possible applications in the treatment of Alzheimer's disease and other forms of dementia.[6][7]
References
- Sternfeld F, Carling RW, Jelley RA, Ladduwahetty T, Merchant KJ, Moore KW, et al. (April 2004). "Selective, orally active gamma-aminobutyric acidA alpha5 receptor inverse agonists as cognition enhancers". Journal of Medicinal Chemistry. 47 (9): 2176–9. doi:10.1021/jm031076j. PMID 15084116.
- Street LJ, Sternfeld F, Jelley RA, Reeve AJ, Carling RW, Moore KW, et al. (July 2004). "Synthesis and biological evaluation of 3-heterocyclyl-7,8,9,10-tetrahydro-(7,10-ethano)-1,2,4-triazolo[3,4-a]phthalazines and analogues as subtype-selective inverse agonists for the GABA(A)alpha5 benzodiazepine binding site". Journal of Medicinal Chemistry. 47 (14): 3642–57. doi:10.1021/jm0407613. PMID 15214791.
- Chambers MS, Atack JR, Broughton HB, Collinson N, Cook S, Dawson GR, et al. (May 2003). "Identification of a novel, selective GABA(A) alpha5 receptor inverse agonist which enhances cognition". Journal of Medicinal Chemistry. 46 (11): 2227–40. doi:10.1021/jm020582q. PMID 12747794.
- Chambers MS, Atack JR, Carling RW, Collinson N, Cook SM, Dawson GR, et al. (November 2004). "An orally bioavailable, functionally selective inverse agonist at the benzodiazepine site of GABAA alpha5 receptors with cognition enhancing properties". Journal of Medicinal Chemistry. 47 (24): 5829–32. doi:10.1021/jm040863t. PMID 15537339.
- Braudeau J, Delatour B, Duchon A, Pereira PL, Dauphinot L, de Chaumont F, et al. (August 2011). "Specific targeting of the GABA-A receptor α5 subtype by a selective inverse agonist restores cognitive deficits in Down syndrome mice". Journal of Psychopharmacology. 25 (8): 1030–42. doi:10.1177/0269881111405366. PMC 3160204. PMID 21693554.
- Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, et al. (March 2006). "An inverse agonist selective for alpha5 subunit-containing GABAA receptors enhances cognition". The Journal of Pharmacology and Experimental Therapeutics. 316 (3): 1335–45. doi:10.1124/jpet.105.092320. PMID 16326923.
- Collinson N, Atack JR, Laughton P, Dawson GR, Stephens DN (November 2006). "An inverse agonist selective for alpha5 subunit-containing GABAA receptors improves encoding and recall but not consolidation in the Morris water maze". Psychopharmacology. 188 (4): 619–28. doi:10.1007/s00213-006-0361-z. PMID 16633803.