17-Phenylandrostenol
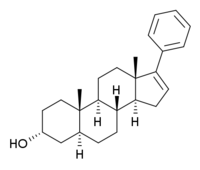
17-Phenylandrostenol (17-PA), or (3α,5α)-17-phenylandrost-16-en-3-ol, is a steroid drug which binds to GABAA receptors. It acts as an antagonist against the sedative effects of neuroactive steroids, but has little effect when administered by itself, and does not block the effects of benzodiazepines or barbiturates.[1][2]
 | |
| Clinical data | |
|---|---|
| Other names | (3α,5α)-17-Phenyl-androst-16-en-3-ol |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C25H34O |
| Molar mass | 350.546 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Chemistry
gollark: But, you know, forever!
gollark: Also, lend us your CB silvers!
gollark: Plot twist: DC is just a testing ground for TJ09's desire sensor system.
gollark: Then when you want a mint or something it's all chronos.
gollark: Basically, see, the cave senses your intention. If you want to catch, say, chrono xenowyrms, no chronos spawn.
See also
References
- Mennerick S, He Y, Jiang X, Manion BD, Wang M, Shute A, Benz A, Evers AS, Covey DF, Zorumski CF (May 2004). "Selective antagonism of 5alpha-reduced neurosteroid effects at GABA(A) receptors". Molecular Pharmacology. 65 (5): 1191–7. doi:10.1124/mol.65.5.1191. PMID 15102947.
- Kelley SP, Alan JK, O'Buckley TK, Mennerick S, Krishnan K, Covey DF, Leslie Morrow A (October 2007). "Antagonism of neurosteroid modulation of native γ-aminobutyric acid receptors by (3α,5α)-17-phenylandrost-16-en-3-ol". European Journal of Pharmacology. 572 (2–3): 94–101. doi:10.1016/j.ejphar.2007.06.028. PMC 2098702. PMID 17658511.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.