Triglyceride
A triglyceride (TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids (from tri- and glyceride).[1] Triglycerides are the main constituents of body fat in humans and other vertebrates, as well as vegetable fat.[2] They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver, and are a major component of human skin oils.[3]
| Types of fats in food |
|---|
| See also |
Many types of triglycerides exist. One classification focuses on saturated and unsaturated types. Saturated fats lack C=C groups. Unsaturated fats feature one or more C=C groups. Unsaturated fats tend to have a lower melting point than saturated analogues. Unsaturated fats often are liquid at room temperature.
Chemical structure
Triglycerides are tri-esters consisting of a glycerol bound to three fatty acid molecules. Alcohols have a hydroxyl (HO–) group. Organic acids have a carboxyl (–COOH) group. Alcohols and organic acids join to form esters. The glycerol molecule has three hydroxyl (HO–) groups and each fatty acid has a carboxyl group (–COOH). In triglycerides, the hydroxyl groups of the glycerol join the carboxyl groups of the fatty acid to form ester bonds:
- HOCH2CH(OH)CH2OH + RCO2H + R′CO2H + R″CO2H → RCO2CH2CH(O2CR′)CH2CO2R″ + 3H2O
The three fatty acids (RCO2H, R′CO2H, R″CO2H in the above equation) are usually different, as many kinds of triglycerides are known. The chain lengths of the fatty acids in naturally occurring triglycerides vary, but most contain 16, 18, or 20 carbon atoms. Natural fatty acids found in plants and animals are typically composed of only even numbers of carbon atoms, reflecting the pathway for their biosynthesis from the two-carbon building-block acetyl CoA. Bacteria, however, possess the ability to synthesise odd- and branched-chain fatty acids. As a result, ruminant animal fat contains odd-numbered fatty acids, such as 15, due to the action of bacteria in the rumen. Many fatty acids are unsaturated; some are polyunsaturated (e.g., those derived from linoleic acid).[4]
Most natural fats contain a complex mixture of individual triglycerides. Because of this, they melt over a broad range of temperatures. Cocoa butter is unusual in that it is composed of only a few triglycerides, derived from palmitic, oleic, and stearic acids in the 1-, 2-, and 3-positions of glycerol, respectively.[4]
Homotriglycerides
The simplest triglycerides are those where the three fatty acids are identical. Their names indicate the fatty acid: stearin derived from stearic acid, palmitin derived from palmitic acid, etc. These compounds can be obtained in three crystalline forms (polymorphs): α, β, and β′, the three forms differing in their melting points.[4][5]
Metabolism
The pancreatic lipase acts at the ester bond, hydrolyzing the bond and "releasing" the fatty acid. In triglyceride form, lipids cannot be absorbed by the duodenum. Fatty acids, monoglycerides (one glycerol, one fatty acid), and some diglycerides are absorbed by the duodenum, once the triglycerides have been broken down.
In the intestine, following the secretion of lipases and bile, triglycerides are split into monoacylglycerol and free fatty acids in a process called lipolysis. They are subsequently moved to absorptive enterocyte cells lining the intestines. The triglycerides are rebuilt in the enterocytes from their fragments and packaged together with cholesterol and proteins to form chylomicrons. These are excreted from the cells and collected by the lymph system and transported to the large vessels near the heart before being mixed into the blood. Various tissues can capture the chylomicrons, releasing the triglycerides to be used as a source of energy. Liver cells can synthesize and store triglycerides. When the body requires fatty acids as an energy source, the hormone glucagon signals the breakdown of the triglycerides by hormone-sensitive lipase to release free fatty acids. As the brain cannot utilize fatty acids as an energy source (unless converted to a ketone),[7] the glycerol component of triglycerides can be converted into glucose, via gluconeogenesis by conversion into dihydroxyacetone phosphate and then into glyceraldehyde 3-phosphate, for brain fuel when it is broken down. Fat cells may also be broken down for that reason if the brain's needs ever outweigh the body's.
Triglycerides cannot pass through cell membranes freely. Special enzymes on the walls of blood vessels called lipoprotein lipases must break down triglycerides into free fatty acids and glycerol. Fatty acids can then be taken up by cells via the fatty acid transporter (FAT).
Triglycerides, as major components of very-low-density lipoprotein (VLDL) and chylomicrons, play an important role in metabolism as energy sources and transporters of dietary fat. They contain more than twice as much energy (approximately 9 kcal/g or 38 kJ/g) as carbohydrates (approximately 4 kcal/g or 17 kJ/g).[8]
Role in disease
In the human body, high levels of triglycerides in the bloodstream have been linked to atherosclerosis, heart disease[9] and stroke.[8] However, the relative negative impact of raised levels of triglycerides compared to that of LDL:HDL ratios is as yet unknown. The risk can be partly accounted for by a strong inverse relationship between triglyceride level and HDL-cholesterol level. But the risk is also due to high triglyceride levels increasing the quantity of small, dense LDL particles.[10]
Guidelines
The National Cholesterol Education Program has set guidelines for triglyceride levels:[11][12]
| Level | Interpretation | |
|---|---|---|
| (mg/dL) | (mmol/L) | |
| < 150 | < 1.70 | Normal range – low risk |
| 150–199 | 1.70–2.25 | Slightly above normal |
| 200–499 | 2.26–5.65 | Some risk |
| 500 or higher | > 5.65 | Very high – high risk |
These levels are tested after fasting 8 to 12 hours. Triglyceride levels remain temporarily higher for a period after eating.
The American Heart Association recommends an optimal triglyceride level of 100 mg/dL (1.1 mmol/L) or lower to improve heart health.[13]
Reducing triglyceride levels
Weight loss and dietary modification are effective first-line lifestyle modification treatments for hypertriglyceridemia.[14] For people with mildly or moderately high levels of triglycerides, lifestyle changes, including weight loss, moderate exercise[15][16] and dietary modification, are recommended.[17] This may include restriction of carbohydrates (specifically fructose)[14] and fat in the diet and the consumption of omega-3 fatty acids[16] from algae, nuts, and seeds.[18] Medications are recommended in those with high levels of triglycerides that are not corrected with the aforementioned lifestyle modifications, with fibrates being recommended first.[17][19][20] Omega-3-carboxylic acids is another prescription drug used to treat very high levels of blood triglycerides.[21]
The decision to treat hypertriglyceridemia with medication depends on the levels and on the presence of other risk factors for cardiovascular disease. Very high levels that would increase the risk of pancreatitis is treated with a drug from the fibrate class. Niacin and omega-3 fatty acids as well as drugs from the statin class may be used in conjunction, with statins being the main medication for moderate hypertriglyceridemia when reduction of cardiovascular risk is required.[17]
Industrial uses
Linseed oil and related oils are important components of useful products used in oil paints and related coatings. Linseed oil is rich in di- and tri-unsaturated fatty acid components, which tend to harden in the presence of oxygen. This heat-producing hardening process is peculiar to these so-called drying oils. It is caused by a polymerization process that begins with oxygen molecules attacking the carbon backbone.
Triglycerides are also split into their components via transesterification during the manufacture of biodiesel. The resulting fatty acid esters can be used as fuel in diesel engines. The glycerin has many uses, such as in the manufacture of food and in the production of pharmaceuticals.
Staining
Staining for fatty acids, triglycerides, lipoproteins, and other lipids is done through the use of lysochromes (fat-soluble dyes). These dyes can allow the qualification of a certain fat of interest by staining the material a specific color. Some examples: Sudan IV, Oil Red O, and Sudan Black B.
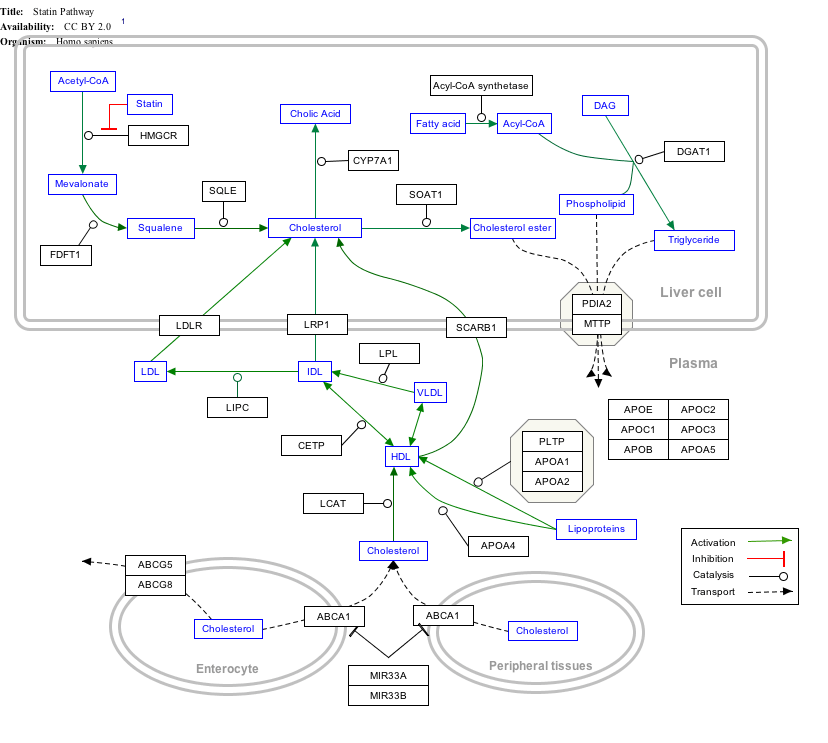
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- The interactive pathway map can be edited at WikiPathways: "Statin_Pathway_WP430".
See also
- AIM-HIGH trial
- Diglyceride acyltransferase, enzyme responsible for triglyceride biosynthesis
- Medium-chain triglycerides
- Lipid profile
- Lipids
- Vertical auto profile
References
- "Nomenclature of Lipids". IUPAC-IUB Commission on Biochemical Nomenclature (CBN). Retrieved 2007-03-08.
- Nelson, D. L.; Cox, M. M. (2000). Lehninger, Principles of Biochemistry (3rd ed.). New York: Worth Publishing. ISBN 1-57259-153-6.
- Lampe, M. A.; Burlingame, A. L.; Whitney, J.; Williams, M. L.; Brown, B. E.; Roitman, E.; Elias, M. (1983). "Human stratum corneum lipids: characterization and regional variations". J. Lipid Res. 24 (2): 120–130. PMID 6833889.
- Alfred Thomas (2002). "Fats and Fatty Oils". Ullmann's Encyclopedia of Industrial Chemistry. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_173. ISBN 3527306730.
- Charbonnet, G. H.; Singleton, W. S. (1947). "Thermal properties of fats and oils". J. Am. Oil Chem. Soc. 24 (5): 140. doi:10.1007/BF02643296.
- Lok, C.M.; Ward, J.P.; van Dorp, D.A. (1976). "The synthesis of Chiral Glycerides starting from D- and L-serine". Chemistry and Physics of Lipids. 16 (2): 115–122. doi:10.1016/0009-3084(76)90003-7. PMID 1269065.
- White, Hayden; Venkatesh, Balasubramanian (2011). "Clinical review: Ketones and brain injury". Critical Care. 15 (2): 219. doi:10.1186/cc10020. PMC 3219306. PMID 21489321.
- Drummond, K. E.; Brefere, L. M. (2014). Nutrition for Foodservice and Culinary Professionals (8th ed.). John Wiley & Sons. ISBN 978-0-470-05242-6.
- "Boston scientists say triglycerides play key role in heart health". The Boston Globe. Retrieved 2014-06-18.
- Ivanova EA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN (2017). "Small Dense Low-Density Lipoprotein as Biomarker for Atherosclerotic Diseases". Oxidative Medicine and Cellular Longevity. 2017: 1273042. doi:10.1155/2017/1273042. PMC 5441126. PMID 28572872.
- "Triglycerides". MedlinePlus. Archived from the original on 26 October 2012. Retrieved 2015-04-23.
- Crawford, H., Micheal. Current Diagnosis & Treatment Cardiology. 3rd ed. McGraw-Hill Medical, 2009. p19
- "What's considered normal?". Triglycerides: Why do they matter?. Mayo Clinic. 28 September 2012.
- Nordestgaard, BG; Varbo, A (August 2014). "Triglycerides and cardiovascular disease". The Lancet. 384 (9943): 626–35. doi:10.1016/S0140-6736(14)61177-6. PMID 25131982.
- GILL, Jason; Sara HERD; Natassa TSETSONIS; Adrianne HARDMAN (Feb 2002). "Are the reductions in triacylglycerol and insulin levels after exercise related?". Clinical Science. 102 (2): 223–231. doi:10.1042/cs20010204. PMID 11834142. Retrieved 2 March 2013.
- Crawford, H., Micheal. Current Diagnosis & Treatment Cardiology. 3rd ed. McGraw-Hill Medical, 2009. p21
- Berglund L, Brunzell JD, Goldberg AC, et al. (September 2012). "Evaluation and treatment of hypertriglyceridemia: an endocrine society clinical practice guideline". J. Clin. Endocrinol. Metab. 97 (9): 2969–89. doi:10.1210/jc.2011-3213. PMC 3431581. PMID 22962670.
- Davidson, Michael H. (28 January 2008). "Pharmacological Therapy for Cardiovascular Disease". In Davidson, Michael H.; Toth, Peter P.; Maki, Kevin C. (eds.). Therapeutic Lipidology. Contemporary Cardiology. Cannon, Christopher P.; Armani, Annemarie M. Totowa, New Jersey: Humana Press, Inc. pp. 141–142. ISBN 978-1-58829-551-4.
- Abourbih S, Filion KB, Joseph L, Schiffrin EL, Rinfret S, Poirier P, Pilote L, Genest J, Eisenberg MJ (2009). "Effect of fibrates on lipid profiles and cardiovascular outcomes: a systematic review". Am J Med. 122 (10): 962.e1–962.e8. doi:10.1016/j.amjmed.2009.03.030. PMID 19698935.
- Jun M, Foote C, Lv J, et al. (2010). "Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis". Lancet. 375 (9729): 1875–1884. doi:10.1016/S0140-6736(10)60656-3. PMID 20462635.
- Blair, HA; Dhillon, S (Oct 2014). "Omega-3 carboxylic acids: a review of its use in patients with severe hypertriglyceridemia". Am J Cardiovasc Drugs. 14 (5): 393–400. doi:10.1007/s40256-014-0090-3. PMID 25234378.