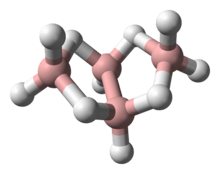
Tetraborane
Tetraborane (systematically named arachno-tetraborane(10)) was the first boron hydride compound to be classified by Stock and Messenez in 1912 and was first isolated by Alfred Stock. It has a relatively low boiling point at 18 °C and is a gas at room temperature. Tetraborane gas is foul smelling and toxic.
 | |
| Names | |
|---|---|
| IUPAC names
tetraborane(10) arachno-B4H10 | |
| Identifiers | |
| ChEBI | |
| ChemSpider | |
| UNII | |
| |
| Properties[1] | |
| B4H10 | |
| Molar mass | 53.32 g/mol |
| Appearance | colourless gas |
| Density | 2.3 kg m−3 (gas) |
| Melting point | −120.8 °C (−185.4 °F; 152.3 K) |
| Boiling point | 18 °C (64 °F; 291 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
History
The class of boranes was elucidated using x-ray diffraction analysis by Lipscomb et. Al. in the 1950s. The x-ray data showed they have 2-electron multicenter bonds. Later, analysis based on high-resolution x-ray data was performed to analyze the charge-density.[2] The comparison of the diffraction data from x-ray diffraction and electron-diffraction gave suspected bond lengths: B1—B2=1.84 A, B1—B3= 1.71 A, <B2B1B4= 98 ̊, B—H=1.19 A, B1—Hμ= 1.33 A, B2—Hμ=1.43 A.[3]
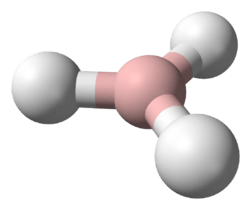
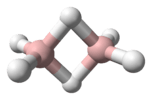
Structure
The “parent” structure of boranes is referred to as closo, but when the second and third vertices are removed the new structure is B4H10 and referred to as arachno.
| Type | formula | notes |
|---|---|---|
| closo− | BnHn2− | No neutral BnHn+2 boranes are known |
| nido− | BnHn+4 | |
| arachno− | BnHn+6 | |
| hypho− | BnHn+8 | only adducts established |
Boron is often called “electron deficient,” because it has three valence electrons, and the standard, carbon, has four. In reality the Boron is stable, and is no way actually deficient of electrons. To solve the issue of “deficiency” the hydrogen bridge or protonated double bonds are introduced to the structure.[4] Each boron is sp3 hybridized and “the configuration of the three hydrogens surrounding borons B1 and B3 is approximately trigonal and suggests approximately tetrahedral hybridization for these borons which would predict bond angles of 120.” However, the Boron arrangements can be classified as fragments of either the icosahedron or the octahedron because the bond angles are actually between 105 ̊ and 90 ̊.[5] The bonding produces a butterfly geometry in the arachno structure. The polyhedral skeletal electron pair theory (PSEPT), originally discovered by Kenneth Wade, are often referred to as Wade’s rules. These rules can be used to count electrons in structures, such as boron hydrides.[6]
Isomers
Scientists are currently working to produce the bis(diboranyl) isomer of the arachno-tetraborane structure. The bis(diboranyl) is expected to have a lower energy at the Hartree-Fock method (HF) level. By using Wurtz reaction or coupling of B2H5I in the presence of the sodium amalgam, scientists believe they have produced the bis(diboranyl) isomer. Once the isomer was created, they tried to discover the mechanism that could then be used to turn the isomer into the arachno-tetraborane structure. Instead of discovering the mechanism, three different pathways have been constructed.
- Path 1: Similar to the dissociative pathway to B3H7 and BH3 proposed by McKee
- Path 2: Concerted pathway over two transition states separated by a local minimum and is different than those suggested by McKee
- Path 3: Involves the penta-coordinated isomers as intermediates… also a concerted pathway
Path two and three are more likely, because they are more energetically favored with energies of 33.1 kcal/mol and 22.7 kcal/mol respectively.[7]
Safety
Because it is easily oxidized it must be kept under vacuum. Tetraborane ignites when it comes in contact with air, oxygen, and nitric acid. Boranes in general including tetraborane have been deemed very toxic and are biologically destructive. A study consisting of small daily exposure of the chemical to rabbits and rats resulted in fatality.[8]
Preparation
Tetraborane can be produced via a reaction between acid and magnesium, aluminum, or beryillium borides. Hydrolysis of magnesium boride, hydrogenation of boron halide (at high temperatures) and the pyrolysis of diborane also produce tetraborane. The hydrolysis of magnesium boride was one of the first reactions to give a high yield(14%) of tetraborane. Phosphoric acid proved to be the most efficient acid (other than hydrochloric and sulfuric acid) in the reaction with magnesium boride. Reduction of boron halides with hydrogen in the presence of metal hydride at high temperatures also produces tetraborane[9]
References
- Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. B-84. ISBN 0-8493-0462-8.
- 5. Forster, Diana. et. Al. (2007). Inorg. Chem. “On the 2-Electron 3-Center B—H—B Bond: Charge Density Determination of Tetraborane(10).”
- 6. Lipscomb, William N. Boron Hydrides. New York: W. A. Benjamin, 1963. Print.
- Grimes, Russel N. "Boron." Advanced Inorganic Chemistry. By F. Albert Cotton, Geoffrey Wilkinson, Carlos A. Murillo, and Manfred Bochmann. 6th ed. N.p.: n.p., 1999. 143-46. Print.
- Lipscomb, William N. Boron Hydrides. New York: W. A. Benjamin, 1963. Print.
- Grimes, Russel N. "Boron." Advanced Inorganic Chemistry. By F. Albert Cotton, Geoffrey Wilkinson, Carlos A. Murillo, and Manfred Bochmann. 6th ed. N.p.: n.p., 1999. 143-46. Print.
- Ramakrishna, Vinutha and Duke, Brian J. (2004). Inorg. Chem. “Can the Bis(diboranyl) Structure of B4H10 Be Observed? The Story Continues.”
- "Archived copy" (PDF). Archived from the original (PDF) on 2011-07-27. Retrieved 2011-05-11.CS1 maint: archived copy as title (link)
- Dain, C. J.; Downs, A. J.; Laurenson, G. S.; Rankin, D. W. H. (1981). "The Molecular Structure of Tetraborane(10) in the Gas Phase as determined by a Joint Analysis of Electron-diffraction and Microwave Data". Journal of the Chemical Society, Dalton Transactions. 1981 (2): 472–477. doi:10.1039/DT9810000472.
External links
- "Boron»tetraborane (10) [WebElements Periodic Table]". Webelements.com. Retrieved 2017-06-07.
- "Linus Pauling Research Notebooks - Special Collections & Archives Research Center". Osulibrary.orst.edu. Retrieved 2017-06-07.