Sodium diacetate
Not to be confused with sodium acetoacetate, a salt of acetoacetic acid or diacetic acid.
 Sodium diacetate | |
| Names | |
|---|---|
| IUPAC name
sodium;diacetate | |
| Other names
Sodium diacetate (anhydrous); Sodium hydrogen acetate; Sodium acid acetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.004.378 |
| MeSH | diacetate sodium diacetate |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H7NaO4 | |
| Molar mass | 142.086 g·mol−1 |
| Appearance | White powder |
| Odor | Acetic acid (vinegar) odor |
| 1 g/mL | |
| Solubility in alcohol | Slightly |
| Solubility in ether | Insoluble |
| Hazards | |
| Safety data sheet | PubChem sodium diacetate LCSS |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements |
H318, H319[2] |
| P264, P280, P305+351+338, P310, P337+313[2] | |
| Inhalation hazard | Irritant[2] |
| Eye hazard | Irritant[2] |
| Flash point | >150 °C (302 °F)[2] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
>2,000 mg/kg (rat, dermal), 5,600 mg/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
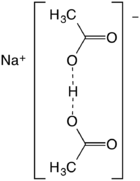
Sodium diacetate is a compound with formula NaH(C
2H
3O
2)
2. It is a salt of acetic acid. It is a colorless solid that is used in seasonings and as an antimicrobial agent.
Preparation and structure
The salt forms upon half-neutralization of acetic acid followed by evaporation of the solution. It can be viewed as the result of homoassociation, an effect that enhances the acidity of acetic acid in concentrated solution:
- 2 CH3CO2H + NaOH → Na+[(CH3CO2)2H]− + H2O
Also described as the sodium acid salt of acetic acid, it is best described as the sodium salt of the hydrogen-bonded anion (CH3CO2)2H−. The O···O distance is about 2.47 angstrom.[3] The species has no significant existence in solution but forms stable crystals.
Applications
As a food additive,[4] it has E number E262 and is used to impart a salt and vinegar flavor.
See also
References
- PubChem. "Sodium diacetate". PubChem. Retrieved 2019-10-24.
- PubChem. "Sodium diacetate". PubChem. Retrieved 2019-10-24.
- Barrow, Michael J.; Currie, Murdoch; Muir, Kenneth W.; Speakman, J. Clare; White, David N, J. "Crystal structures of some acid salts of monobasic acids. XVII. Structure of sodium hydrogen diacetate, redetermined by neutron diffraction" Journal of the Chemical Society, Perkin Transactions 2: Physical Organic Chemistry 1975, pp. 15-18. doi:10.1039/P29750000015
- Peter J. Taormina "Implications of Salt and Sodium Reduction on Microbial Food Safety" in Critical Reviews in Food Science and Nutrition, 2010, vol. 50, 209-227. doi:10.1080/10408391003626207
Acetyl halides and salts of the acetate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 |
Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||