Short-term effects of alcohol consumption
The short-term effects of alcohol (more specifically ethanol) consumption – due to drinking beer, wine, distilled spirits or other alcoholic beverages – range from a decrease in anxiety and motor skills and euphoria at lower doses to intoxication (drunkenness), stupor, unconsciousness, anterograde amnesia (memory "blackouts"), and central nervous system depression at higher doses. Cell membranes are highly permeable to alcohol, so once alcohol is in the bloodstream, it can diffuse into nearly every cell in the body.
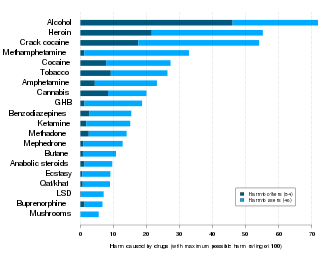
The concentration of alcohol in blood is measured via blood alcohol content (BAC). The amount and circumstances of consumption play a large role in determining the extent of intoxication; for example, eating a heavy meal before alcohol consumption causes alcohol to absorb more slowly.[1] The amount of alcohol consumed largely determines the extent of hangovers, although hydration also plays a role. After excessive drinking, stupor and unconsciousness both can occur. Extreme levels of consumption can cause alcohol poisoning and death; in fact, a concentration in the blood stream of 0.36% will kill half of those affected.[2][3][4] Alcohol may also cause death indirectly, by asphyxiation from vomiting.
Alcohol can greatly exacerbate sleep problems. During abstinence, residual disruptions in sleep regularity and sleep patterns are the greatest predictors of relapse.[5]
Effects by dosage
The definition of a unit of alcohol ranges between 8 and 14 grams of pure alcohol/ethanol depending on the country.[6] There is no agreement on definitions of a low, moderate or high dose of alcohol either. The U.S. National Institute on Alcohol Abuse and Alcoholism defines a moderate dose as alcohol intake up to two standard drinks or 28 grams for men and one standard drink or 14 grams for women.[7] The immediate effect of alcohol depends on the drinker's blood alcohol concentration (BAC). BAC can be different for each person depending on their age, sex, pre-existing health condition, even if they drink the same amount of alcohol.[8]
Different BACs have different effects. The following lists describe the common effects of alcohol on the body depending on the BAC. However, tolerance varies considerably between individuals, as does individual response to a given dosage; the effects of alcohol differ widely between people. Hence in this context, BAC percentages are just estimates used for illustrative purposes.
| BAC (% by vol.) | SI units (mmol/l) | Behavior | Impairment |
|---|---|---|---|
| 0.001–0.029 | 0.22–6.3 |
|
|
| 0.030–0.059 | 6.5–12.8 |
|
|
| 0.060–0.099 | 13.0–21.5 |
| |
| 0.100–0.199 | 21.7–43.3 |
| |
| 0.200–0.299 | 43.4–64.9 |
|
|
| 0.300–0.399 | 65.1–86.6 |
|
|
| 0.400–0.500 | 86.80–108.5 |
|
|
| >0.50 | >108.5 |
|
Grand Rapids Dip
Studies suggest that a BAC of 0.01–0.04% slightly lowers risk of being in an vehicle accident when compared to a BAC of 0.00%, referred to as the Grand Rapids Effect or Grand Rapids Dip.[10][11][12] Some literature has attributed the Grand Rapids Effect to erroneous data or asserted (without support) that it was possibly due to drivers exerting extra caution at low BAC levels or due to "experience" in drinking. Other explanations are that this effect is at least in part the blocking effect of ethanol excitotoxicity and the effect of alcohol in essential tremor and other movement disorders,[13] but this remains speculative.
Moderate doses
Ethanol inhibits the ability of glutamate to open the cation channel associated with the N-methyl-D-aspartate (NMDA) subtype of glutamate receptors. Stimulated areas include the cortex, hippocampus, and nucleus accumbens, which are all responsible for both thinking and pleasure seeking. Another one of alcohol's agreeable effects is body relaxation, which is possibly caused by neurons transmitting electrical signals in an alpha waves-pattern; such waves are actually observed (with the aid of EEGs) whenever the body is relaxed.
Short-term effects of alcohol include the risk of injuries, violence, and fetal damage.[14] Alcohol has also been linked with lowered inhibitions, although it is unclear as to what degree this is chemical or psychological as studies with placebos can often duplicate the social effects of alcohol at either low or moderate doses. Some studies have suggested that intoxicated people have much greater control over their behavior than is generally recognized, though they have a reduced ability to evaluate the consequences of their behavior.[15] Behavioral changes associated with drunkenness are, to some degree, contextual.[16][17]
Areas of the brain that are responsible for planning and motor learning are sharpened. A related effect, which is caused by even low levels of alcohol, is the tendency for people to become more animated in speech and movement. This is caused by increased metabolism in areas of the brain associated with movement, such as the nigrostriatal pathway. This causes reward systems in the brain to become more active, which may induce certain individuals to behave in an uncharacteristically loud and cheerful manner.
Alcohol has been known to mitigate the production of antidiuretic hormone, which is a hormone that acts on the kidney to favor water reabsorption in the kidneys during filtration. This occurs because alcohol confuses osmoreceptors in the hypothalamus, which relay osmotic pressure information to the posterior pituitary, the site of antidiuretic hormone release. Alcohol causes the osmoreceptors to signal that there is low osmotic pressure in the blood, which triggers an inhibition of the antidiuretic hormone. As a consequence, one's kidneys are no longer able to reabsorb as much water as they should be absorbing, therefore creating excessive volumes of urine and the subsequent overall dehydration.
Excessive doses
Acute alcohol intoxication through excessive doses in general causes short- or long-term health effects. NMDA receptors become unresponsive, slowing areas of the brain for which they are responsible. Contributing to this effect is the activity that alcohol induces in the gamma-aminobutyric acid (GABA) system. The GABA system is known to inhibit activity in the brain. GABA could also be responsible for causing the memory impairment that many people experience. It has been asserted that GABA signals interfere with both the registration and the consolidation stages of memory formation. As the GABA system is found in the hippocampus (among other areas in the CNS), which is thought to play a large role in memory formation, this is thought to be possible.
Anterograde amnesia, colloquially referred to as "blacking out", is another symptom of heavy drinking. This is the loss of memory during and after an episode of drinking. When alcohol is consumed at a rapid rate, the point at which most healthy people's long-term memory creation starts failing usually occurs at approximately 0.20% BAC, but it can be reached as low as 0.14% BAC for inexperienced drinkers.
Another classic finding of alcohol intoxication is ataxia, in its appendicular, gait, and truncal forms. Appendicular ataxia results in jerky, uncoordinated movements of the limbs, as if each muscle were working independently from the others. Truncal ataxia results in postural instability; gait instability is manifested as a disorderly, wide-based gait with inconsistent foot positioning. Ataxia causes the observation that drunk people are clumsy, sway back and forth, and often fall down. It is presumed to be due to alcohol's effect on the cerebellum.
Mellanby effect
The Mellanby effect is the phenomenon that the behavioral impairment due to alcohol is less, at the same BAC, when the BAC is decreasing than when it is increasing.[18] In other words, at a given point on the upward slope of BAC (while drinking), impairment is greater than at a given point on the downward slope (after drinking), even though the BAC is the same at the two points. This effect was confirmed in a 2017 meta-analysis.[19]
Effect on different population
Based on sex
Alcohol affects male and female differently because of difference in body fat percentage and water content. On average, for equal body weight, women have a higher body fat percentage than men. Since alcohol is absorbed into body water content and men have more water in their bodies than women, for women there will be a higher blood alcohol concentration from same amount of alcohol consumption.[20] Women are also thought to have less alcohol dehydrogenase (ADH) enzyme which is required to break down alcohol.[8] That is why the drinking guidelines are different for men and women.[21]
Based on genetic variation
Alcohol metabolism depends on the enzymes alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH).[22] Genetic variants of the genes coding for these enzymes can affect the rate of alcohol metabolism. Some ADH gene variants lead to higher metabolic activity, resulting in the accumulation of acetaldehyde, whereas, a null allele in ALDH2 causes an accumulation of acetaldehyde by preventing its catabolism to acetate.[23] The genetic variants of these enzymes can explain the differences in the alcohol metabolism in different races. The different isoforms of ADH showed protection against alcoholic disorders in Han Chinese and Japanese (due to presence of ADH1B*2 ) and in African (due to presence of ADH1B*3).[24] On the other hand, presence of ALDH2*2 in East Asians (a variant of the ALDH gene), can cause blood acetaldehyde levels of 30 to 75 μM or higher, which is more than 10 times the normal level. The excess amount of blood aldehyde produce facial flushing, nausea, rapid heartbeat, and other adverse effects.[25][26] Presence of these alleles causes rapid conversion of alcohol to acetaldehyde which can be toxic in large amount. So, the East Asians and Africans feel the adverse effects of alcohol early and stop drinking. For Caucasians, ADH1B*1 allele is the most prevalent allele which causes slower conversion of alcohol to acetaldehyde and it makes them more vulnerable to alcohol use disorders.[27]
Allergic reaction-like symptoms
Humans metabolize ethanol primarily through NAD+-dependent alcohol dehydrogenase (ADH) class I enzymes (i.e. ADH1A, ADH1B, and ADH1C) to acetaldehyde and then metabolize acetaldehyde primarily by NAD2-dependent aldehyde dehydrogenase 2 (ALDH2) to acetic acid.[28][29] Eastern Asians reportedly have a deficiency in acetaldehyde metabolism in a surprisingly high percentage (approaching 50%) of their populations. The issue has been most thoroughly investigated in native Japanese where persons with a single-nucleotide polymorphism (SNP) variant allele of the ALDH2 gene were found; the variant allele, encodes lysine (lys) instead of glutamic acid (glu) at amino acid 487; this renders the enzyme essentially inactive in metabolizing acetaldehyde to acetic acid.[30][31] The variant allele is variously termed glu487lys, ALDH2*2, and ALDH2*504lys. In the overall Japanese population, about 57% of individuals are homozygous for the normal allele (sometimes termed ALDH2*1), 40% are heterozygous for glu487lys, and 3% are homozygous for glu487lys.[31] Since ALDH2 assembles and functions as a tetramer and since ALDH2 tetramers containing one or more glu487lys proteins are also essentially inactive (i.e. the variant allele behaves as a dominant negative), homozygote individuals for glu487lys have undetectable while heterozygote individuals for glu487lys have little ALDH2 activity.[32] In consequence, Japanese individuals homozygous or, to only a slightly lesser extent, heterozygous for glu487lys metabolize ethanol to acetaldehyde normally but metabolize acetaldehyde poorly and are susceptible to a set of adverse responses to the ingestion of, and sometimes even the fumes from, ethanol and ethanol-containing beverages; these responses include the transient accumulation of acetaldehyde in blood and tissues; facial flushing (i.e. the "oriental flushing syndrome" or Alcohol flush reaction), urticaria, systemic dermatitis, and alcohol-induced respiratory reactions (i.e. rhinitis and, primarily in patients with a history of asthma, mild to moderately bronchoconstriction exacerbations of their asthmatic disease.[33] These allergic reaction-like symptoms, which typically occur within 30–60 minutes of ingesting alcoholic beverages, do not appear to reflect the operation of classical IgE- or T cell-related allergen-induced reactions but rather are due, at least in large part, to the action of acetaldehyde in stimulating tissues to release histamine, the probable evoker of these symptoms.[33][34]
The percentages of glu487lys heterozygous plus homozygous genotypes are about 35% in native Caboclo of Brazil, 30% in Chinese, 28% in Koreans, 11% in Thai people, 7% in Malaysians, 3% in natives of India, 3% in Hungarians, and 1% in Filipinos; percentages are essentially 0 in individuals of Native African descent, Caucasians of Western European descent, Turks, Australian Aborigines, Australians of Western European descent, Swedish Lapps, and Alaskan Eskimos.[34][35] The prevalence of ethanol-induced allergic symptoms in 0 or low levels of glu487lys genotypes commonly ranges above 5%. These "ethanol reactors" may have other gene-based abnormalities that cause the accumulation of acetaldehyde following the ingestion of ethanol or ethanol-containing beverages. For example, the surveyed incidence of self-reported ethanol-induced flushing reactions in Scandinavians living in Copenhagen as well as Australians of European descent is about 16% in individuals homozygous for the "normal" ADH1B gene but runs to ~23% in individuals with the ADH1-Arg48His SNP variant; in vitro, this variant metabolizes ethanol rapidly and in humans, it is proposed, may form acetaldehyde at levels that exceed the capacity of ALDH2 to metabolize.[34][36] Notwithstanding such considerations, experts suggest that the large proportion of alcoholic beverage-induced allergic-like symptoms in populations with a low incidence of the glu487lys genotype reflect true allergic reactions to the natural and/or contaminating allergens particularly those in wines and to a lesser extent beers.[33]
Pathophysiology
At low or moderate doses, alcohol acts primarily as a positive allosteric modulator of GABAA. Alcohol binds to several different subtypes of GABAA, but not to others. The main subtypes responsible for the subjective effects of alcohol are the α1β3γ2, α5β3γ2, α4β3δ and α6β3δ subtypes, although other subtypes such as α2β3γ2 and α3β3γ2 are also affected. Activation of these receptors causes most of the effects of alcohol such as relaxation and relief from anxiety, sedation, ataxia and increase in appetite and lowering of inhibitions that can cause a tendency toward violence in some people.[37][38][39][40][41][42][43]
Alcohol has a powerful effect on glutamate as well. Alcohol decreases glutamate's ability to bind with NMDA and acts as an antagonist of the NMDA receptor, which plays a critical role in LTP by allowing Ca2+ to enter the cell. These inhibitory effects are thought to be responsible for the "memory blanks" that can occur at levels as low as 0.03% blood level. In addition, reduced glutamate release in the dorsal hippocampus has been linked to spatial memory loss. Chronic alcohol users experience an upregulation of NMDA receptors because the brain is attempting to reestablish homeostasis. When a chronic alcohol user stops drinking for more than 10 hours, apoptosis can occur due to excitotoxicity. The seizures experienced during alcohol abstinence are thought to be a result of this NMDA upregulation. Alteration of NMDA receptor numbers in chronic alcoholics is likely to be responsible for some of the symptoms seen in delirium tremens during severe alcohol withdrawal, such as delirium and hallucinations. Other targets such as sodium channels can also be affected by high doses of alcohol, and alteration in the numbers of these channels in chronic alcoholics is likely to be responsible for as well as other effects such as cardiac arrhythmia. Other targets that are affected by alcohol include cannabinoid, opioid and dopamine receptors, although it is unclear whether alcohol affects these directly or if they are affected by downstream consequences of the GABA/NMDA effects. People with a family history of alcoholism may exhibit genetic differences in the response of their NMDA glutamate receptors as well as the ratios of GABAA subtypes in their brain.[44][45][46][47][48][49][50] Alcohol inhibits sodium-potassium pumps in the cerebellum and this is likely how it corrupts cerebellar computation and body co-ordination.[51][52]
Contrary to popular belief, research suggests that acute exposure to alcohol is not neurotoxic in adults and actually prevents NMDA antagonist-induced neurotoxicity.[53]
Alcohol and sleep
Low doses of alcohol (one 360 ml (13 imp fl oz; 12 US fl oz) beer) appear to increase total sleep time and reduce awakening during the night. The sleep-promoting benefits of alcohol dissipate at moderate and higher doses of alcohol.[54] Previous experience with alcohol also influences the extent to which alcohol positively or negatively affects sleep. Under free-choice conditions, in which subjects chose between drinking alcohol or water, inexperienced drinkers were sedated while experienced drinkers were stimulated following alcohol consumption.[55] In insomniacs, moderate doses of alcohol improve sleep maintenance.[56]
Moderate alcohol consumption 30–60 minutes before sleep, although decreasing, disrupts sleep architecture. Rebound effects occur once the alcohol has been largely metabolized, causing late night disruptions in sleep maintenance. Under conditions of moderate alcohol consumption where blood alcohol levels average 0.06–0.08 percent and decrease 0.01–0.02 percent per hour, an alcohol clearance rate of 4–5 hours would coincide with disruptions in sleep maintenance in the second half of an 8-hour sleep episode. In terms of sleep architecture, moderate doses of alcohol facilitate "rebounds" in rapid eye movement (REM) following suppression in REM and stage 1 sleep in the first half of an 8-hour sleep episode, REM and stage 1 sleep increase well beyond baseline in the second half. Moderate doses of alcohol also very quickly increase slow wave sleep (SWS) in the first half of an 8-hour sleep episode. Enhancements in REM sleep and SWS following moderate alcohol consumption are mediated by reductions in glutamatergic activity by adenosine in the central nervous system. In addition, tolerance to changes in sleep maintenance and sleep architecture develops within 3 days of alcohol consumption before bedtime.
Alcohol consumption and balance
Alcohol can affect balance by altering the viscosity of the endolymph within the otolithic membrane, the fluid inside the semicircular canals inside the ear. The endolymph surrounds the ampullary cupula which contains hair cells within the semicircular canals. When the head is tilted, the endolymph flows and moves the cupula. The hair cells then bend and send signals to the brain indicating the direction in which the head is tilted. By changing the viscosity of the endolymph to become less dense when alcohol enters the system, the hair cells can move more easily within the ear, which sends the signal to the brain and results in exaggerated and overcompensated movements of body. This can also result in vertigo, or "the spins."[57]
Alcohol and postprandial triglycerides
Alcohol taken with a meal increases and prolongs postprandial triglyceridemia. This is true despite the observation that the relationship between alcohol consumption and triglyceridemia is "J-shaped," meaning that fasting triglycerides concentration is lower in people who drink 10–20 g/alcohol a day compared to people who either abstain from alcohol or who drink more per day.[58]
Alcohol and blood pressure
A systematic review reported that alcohol has bi-phasic effect on blood pressure. Both systolic and diastolic blood pressure fell when they were measured couple of hours after alcohol consumption. However, the longer term measurement (20 hours average) showed a modest but statistically significant increase in blood pressure: a 2.7 mmHg rise in systolic blood pressure and 1.4 mmHg rise in diastolic blood pressure.[59] A Cochrane systematic review based on only randomized controlled trials which investigates the acute effect of alcohol consumption in healthy and hypertensive adults is in progress.[60]
See also
- Alcohol and health
- Long-term effects of alcohol consumption
References
- Horowitz M, Maddox A, Bochner M, et al. (August 1989). "Relationships between gastric emptying of solid and caloric liquid meals and alcohol absorption". Am. J. Physiol. 257 (2 Pt 1): G291–8. doi:10.1152/ajpgi.1989.257.2.G291. PMID 2764113.
- Jones, A. W. et al. Comparison of Blood-Ethanol Concentration in Deaths Attributed to Acute Alcohol Poisoning and Chronic Alcoholism. Journal of Forensic Sciences 48(4):874-9 · July 2003
- Alcohol Awareness Page
- Carleton College: Wellness Center: Blood Alcohol Concentration (BAC) Archived 14 September 2009 at the Wayback Machine
- Feige B, Scaal S, Hornyak M, Gann H, Riemann D (January 2007). "Sleep electroencephalographic spectral power after withdrawal from alcohol in alcohol-dependent patients". Alcohol. Clin. Exp. Res. 31 (1): 19–27. doi:10.1111/j.1530-0277.2006.00260.x. PMID 17207097.
- "Sensible Drinking Guidelines" (PDF). Alcohol in Moderation. 2018.
- "Drinking Levels Defined". National Institute on Alcohol Abuse and Alcoholism. 14 September 2011.
- Cederbaum, AI (2012). "Alcohol metabolism". Clinics in Liver Disease. 16 (4): 667–685. doi:10.1016/j.cld.2012.08.002. PMC 3484320. PMID 23101976.
- A hybridizing of effects as described at Alcohol's Effects Archived May 5, 2007, at the Wayback Machine from Virginia Tech and Federal Aviation Regulation (CFR) 91.17: Alcohol and Flying (hosted on FlightPhysical.com)
- Grand Rapids Effects Revisited: Accidents, Alcohol and Risk, H.-P. Krüger, J. Kazenwadel and M. Vollrath, Center for Traffic Sciences, University of Wuerzburg, Röntgenring 11, D-97070 Würzburg, Germany
- NTSB (US) report on Grand Rapids Effect
- Robert F. Borkenstein papers, 1928–2002, Indiana U. The role of the drinking driver in traffic accidents (Researchgate link)
- Mostile G, Jankovic J (2010). "Alcohol in essential tremor and other movement disorders". Movement Disorders. 25 (14): 2274–2284. doi:10.1002/mds.23240. PMID 20721919.
- Andréasson, S.; Allebeck, P. (28 February – 6 March 2005). "[Alcohol as medication is no good. More risks than benefits according to a survey of current knowledge]". Lakartidningen. 102 (9): 632–7. PMID 15804034.
- Grattan KE, Vogel-Sprott M (February 2001). "Maintaining intentional control of behavior under alcohol". Alcohol. Clin. Exp. Res. 25 (2): 192–7. doi:10.1111/j.1530-0277.2001.tb02198.x. PMID 11236832.
- Grant NK, Macdonald TK (2005). "Can alcohol lead to inhibition or disinhibition? Applying alcohol myopia to animal experimentation". Alcohol Alcohol. 40 (5): 373–8. doi:10.1093/alcalc/agh177. PMID 15996970.
- "Social and Cultural Aspects of Drinking - Culture Chemistry and Consequences".
- Moskowitz H, Henderson R, Daily J (1979). "The Mellanby Effect in Moderate and Heavy Drinkers" (PDF). International Conference on Alcohol, Drugs and Traffic Safety: 184–189.
- Holland, MG; Ferner, RE (2017). "A systematic review of the evidence for acute tolerance to alcohol – the "Mellanby effect"" (PDF). Clinical Toxicology. 55 (6): 545–556. doi:10.1080/15563650.2017.1296576. ISSN 1556-9519. PMID 28277803.
- "What Is a Safe Level of Drinking?". alcohol.org. 15 March 2019. Retrieved 5 April 2019.
- "Drinking Guidelines". Canadian Centre on Substance Use and Addiction.
- Edenberg HJ, McClintick JN (2018). "Alcohol Dehydrogenases, Aldehyde Dehydrogenases, and Alcohol Use Disorders: A Critical Review". Alcohol. Clin. Exp. Res. 42 (12): 2281–2297. doi:10.1111/acer.13904. PMC 6286250. PMID 30320893.
- Chen, Hui-Ju; Tian, H; Edenberg, HJ (2005). "Natural haplotypes in the regulatory sequences affect human alcohol dehydrogenase 1C (ADH1C) gene expression". Human Mutation. 25 (2): 150–155. doi:10.1002/humu.20127. PMID 15643610.
- Thomasson, HR; Beard, JD; Li, TK (1995). "ADH2 gene polymorphisms are determinants of alcohol pharmacokinetics". Alcoholism, Clinical and Experimental Research. 19 (6): 1494–1499. doi:10.1111/j.1530-0277.1995.tb01013.x. PMID 8749816.
- Enomoto, N; Takase, S; Yasuhara, M; Takada, A (1991). "Acetaldehyde metabolism in different aldehyde dehydrogenase-2 genotypes". Alcoholism, Clinical and Experimental Research. 15 (1): 141–144. doi:10.1111/j.1530-0277.1991.tb00532.x. PMID 2024727.
- Peng, Q; Gizer, IR; Libiger, O; Bizon, C; Wilhelmsen, KC; Schork, NJ; Ehlers, CL (2014). "Association and ancestry analysis of sequence variants in ADH and ALDH using alcohol-related phenotypes in a Native American community sample". American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 165 (8): 673–683. doi:10.1002/ajmg.b.32272. PMC 4364382. PMID 25270064.
- Dodge NC, Jacobson JL, Jacobson SW (2014). "Protective effects of the alcohol dehydrogenase-ADH1B*3 allele on attention and behavior problems in adolescents exposed to alcohol during pregnancy". Neurotoxicol Teratol. 41: 43–50. doi:10.1016/j.ntt.2013.11.003. PMC 3943945. PMID 24263126.
- Ann Ist Super Sanita. 2006;42(1):8–16
- Crabb DW (2004). "Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology". Proceedings of the Nutrition Society. 63: 49–63. doi:10.1079/PNS2003327. PMID 15099407.
- Wolff PH (1972). "Ethnic Differences in Alcohol Sensitivity". Science. 175: 449–450. doi:10.1126/science.175.4020.449.
- Takao A (1998). "Correlation between alcohol-induced asthma and acetaldehyde dehydrogenase-2 genotype☆☆☆★". Journal of Allergy and Clinical Immunology. 101: 576–580. doi:10.1016/s0091-6749(98)70162-9. PMID 9600491.
- Koppaka V (2012). "Aldehyde Dehydrogenase Inhibitors: a Comprehensive Review of the Pharmacology, Mechanism of Action, Substrate Specificity, and Clinical Application". Pharmacological Reviews. 64: 520–539. doi:10.1124/pr.111.005538. PMC 3400832. PMID 22544865.
- Adams KE, Rans TS (2013). "Adverse reactions to alcohol and alcoholic beverages". Ann. Allergy Asthma Immunol. 111 (6): 439–445. doi:10.1016/j.anai.2013.09.016. PMID 24267355.
- Macgregor S, Lind PA, Bucholz KK, Hansell NK, Madden PA, Richter MM, Montgomery GW, Martin NG, Heath AC, Whitfield JB (2009). "Associations of ADH and ALDH2 gene variation with self report alcohol reactions, consumption and dependence: an integrated analysis". Hum. Mol. Genet. 18 (3): 580–593. doi:10.1093/hmg/ddn372. PMC 2722191. PMID 18996923.
- Goedde HW, Agarwal DP, Fritze G, Meier-Tackmann D, Singh S, Beckmann G, Bhatia K, Chen LZ, Fang B, Lisker R (1992). "Distribution of ADH2 and ALDH2 genotypes in different populations". Hum Genet. 88 (3): 344–346. doi:10.1007/bf00197271. PMID 1733836.
- Linneberg A, Fenger RV, Husemoen LL, Vidal C, Vizcaino L, Gonzalez-Quintela A (2010). "Immunoglobulin E sensitization to cross-reactive carbohydrate determinants: epidemiological study of clinical relevance and role of alcohol consumption". Int. Arch. Allergy Immunol. 153 (1): 86–94. doi:10.1159/000301583. PMID 20357489.
- Huang Q, He X, Ma C, et al. (2000). "Pharmacophore/receptor models for GABAA/BzR subtypes (α1β3γ2, α5β3γ2, and α6β3γ2) via a comprehensive ligand-mapping approach". J. Med. Chem. 43 (1): 71–95. doi:10.1021/jm990341r. PMID 10633039.
- Platt DM, Duggan A, Spealman RD, et al. (2005). "Contribution of α1GABAA and α5GABAA receptor subtypes to the discriminative stimulus effects of ethanol in squirrel monkeys". J. Pharmacol. Exp. Ther. 313 (2): 658–667. doi:10.1124/jpet.104.080275. PMID 15650112.
- Duke AN, Platt DM, Cook JM, et al. (2006). "Enhanced sucrose pellet consumption induced by benzodiazepine-type drugs in squirrel monkeys: role of GABAA receptor subtypes". Psychopharmacology. 187 (3): 321–330. doi:10.1007/s00213-006-0431-2. PMID 16783540.
- Wallner M, Hanchar HJ, Olsen RW (2006). "Low-dose alcohol actions on α4β3δ GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513". Proc Natl Acad Sci USA. 103 (22): 8540–8545. Bibcode:2006PNAS..103.8540W. doi:10.1073/pnas.0600194103. PMC 1482527. PMID 16698930.
- Mehta AK, Ticku MK (1988). "Ethanol potentiation of GABAergic transmission in cultured spinal cord neurons involves gamma-aminobutyric acidA-gated chloride channels". J. Pharmacol. Exp. Ther. 246 (2): 558–564. PMID 2457076.
- Becker HC, Anton RF (1989). "The benzodiazepine receptor inverse agonist RO15-4513 exacerbates, but does not precipitate, ethanol withdrawal in mice". Pharmacol Biochem Behav. 32 (1): 163–167. doi:10.1016/0091-3057(89)90227-X. PMID 2543989.
- Hanchar HJ, Chutsrinopkun P, Meera P, et al. (2006). "Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to α4/6β3δ GABAA". Proc Natl Acad Sci USA. 103 (22): 8546–8551. Bibcode:2006PNAS..103.8546H. doi:10.1073/pnas.0509903103. PMC 1482528. PMID 16581914.
- Shimizu, A. Matsubara, K. Uezono, T. Kimura, K. Shiono, H. "Reduced dorsal hippocampal glutamate release significantly correlates with the spatial memory deficits produced by benzodiazepines and ethanol". Neuroscience. P 701-706
- Petrakis IL, Limoncelli D, Gueorguieva R, et al. (2004). "Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism". Am J Psychiatry. 161 (10): 1776–1782. doi:10.1176/appi.ajp.161.10.1776. PMID 15465973.
- Nutt DJ (2006). "Alcohol alternatives—a goal for psychopharmacology?". J. Psychopharmacol. (Oxford). 20 (3): 318–320. doi:10.1177/0269881106063042. PMID 16574703.
- Qiang M, Denny AD, Ticku MK (2007). "Chronic intermittent ethanol treatment selectively alters N-methyl-D-aspartate receptor subunit surface expression in cultured cortical neurons". Mol Pharmacol. 72 (1): 95–102. doi:10.1124/mol.106.033043. PMID 17440117.
- Dodd PR, Buckley ST, Eckert AL, Foley PF, Innes DJ (2006). "Genes and gene expression in the brains of human alcoholics". Ann N Y Acad Sci. 1074 (1): 104–115. Bibcode:2006NYASA1074..104D. doi:10.1196/annals.1369.010. PMID 17105908.
- Klein G, Gardiwal A, Schaefer A, Panning B, Breitmeier D (2007). "Effect of ethanol on cardiac single sodium channel gating". Forensic Sci Int. 171 (2–3): 131–135. doi:10.1016/j.forsciint.2006.10.012. PMID 17129694.
- Shiraishi M, Harris RA (2004). "Effects of alcohols and anesthetics on recombinant voltage-gated Na+ channels". J. Pharmacol. Exp. Ther. 309 (3): 987–994. doi:10.1124/jpet.103.064063. PMID 14978193.
- Forrest MD (2015). "Simulation of alcohol action upon a detailed Purkinje neuron model and a simpler surrogate model that runs >400 times faster". BMC Neuroscience. 16 (27): 27. doi:10.1186/s12868-015-0162-6. PMC 4417229. PMID 25928094.
- Forrest, Michael (April 2015). "The neuroscience reason we fall over when drunk". Science 2.0. Retrieved 24 June 2015.
- Farber, NB; Heinkel, C; Dribben, WH; Nemmers, B; Jiang, X (2004). "In the adult CNS, ethanol prevents rather than produces NMDA antagonist-induced neurotoxicity". Brain Research. 1028 (1): 66–74. doi:10.1016/j.brainres.2004.08.065. PMID 15518643.
- Stone BM (1980). "Sleep and low doses of alcohol". Electroencephalogr Clin Neurophysiol. 48 (6): 706–709. doi:10.1016/0013-4694(80)90427-7. PMID 6155259.
- Schuckit MA (1994). "Low level of response to alcohol as a predictor of future alcoholism". Am J Psychiatry. 151 (2): 184–189. doi:10.1176/ajp.151.2.184. PMID 8296886.
- Roehrs T, Papineau K, Rosenthal L, Roth T (1999). "Ethanol as a hypnotic in insomniacs: self administration and effects on sleep and mood". Neuropsychopharmacology. 20 (3): 279–286. doi:10.1016/S0893-133X(98)00068-2. PMID 10063488.
- , Why does my head spin when I'm drunk? Rachel Nuwer, 8 Feb 2011, Scienceline.
- Kovář J, Zemánková K (2015). "Moderate alcohol consumption and triglyceridemia". Physiol Res. 64 Suppl 3: S371–S375. PMID 26680670.
- McFadden CB, Brensinger CM, Berlin JA, Townsend RR (2005). "Systematic review of the effect of daily alcohol intake on blood pressure". Am J Hypertens. 18 (2 Pt 1): 276–286. doi:10.1016/j.amjhyper.2004.07.020. PMID 15752957.
- Tasnim, S; Tang, C; Wright, JM (1 September 2017). "Effect of alcohol on blood pressure". Cochrane Database of Systematic Reviews. 2017 (9): CD012787. doi:10.1002/14651858.CD012787. PMC 6483609.
External links
- Global Status Report on Alcohol 2004 by the World Health Organization.
- Heberlein, Ulrike; Wolf, Fred W.; Rothenfluh, Adrian; Guarnieri, Douglas J. (8 January 2004). "Molecular Genetic Analysis of Ethanol Intoxication in Drosophila Melanogaster". Integrative and Comparative Biology. 44 (4): 269–74. CiteSeerX 10.1.1.536.262. doi:10.1093/icb/44.4.269. PMID 21676709.