Elasmobranchii
Elasmobranchii (/ɪˌlæzməˈbræŋkiaɪ/[8]) is a subclass of Chondrichthyes or cartilaginous fish, including the sharks (superorder Selachii) and the rays, skates, and sawfish (superorder Batoidea). Members of this subclass are characterised by having four (tawny nurse sharks have 4 pairs of gills) to seven pairs of gill clefts opening individually to the exterior, rigid dorsal fins and small placoid scales on the skin. The teeth are in several series; the upper jaw is not fused to the cranium, and the lower jaw is articulated with the upper. The details of this jaw anatomy vary between species, and help distinguish the different elasmobranch clades. The pelvic fins in males are modified to create claspers for the transfer of sperm. There is no swim bladder; instead, these fish maintain buoyancy with large livers rich in oil.
| Elasmobranchii | |
|---|---|
 | |
| Great white shark (Carcharodon carcharias) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Chondrichthyes |
| Subclass: | Elasmobranchii Bonaparte, 1838 |
| Superorders | |
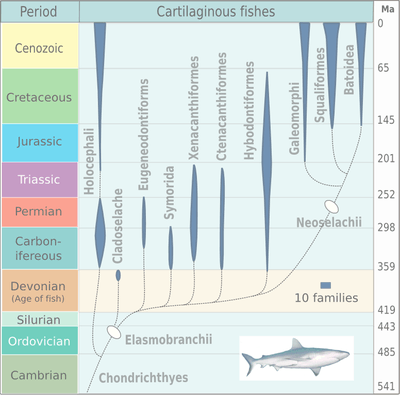
The earliest elasmobranch fossils came from the Devonian and many surviving orders date back to the Cretaceous, or even earlier. Many species became extinct during the Permian and there was a burst of adaptive radiation during the Jurassic.
Description
Elasmobranchii is one of the two subclasses of cartilaginous fish in the class Chondrichthyes, the other being Holocephali (chimaeras).
Members of the elasmobranchii subclass have no swim bladders, five to seven pairs of gill clefts opening individually to the exterior, rigid dorsal fins, and small placoid scales. The teeth are in several series; the upper jaw is not fused to the cranium, and the lower jaw is articulated with the upper.
Extant elasmobranchs exhibit several archetypal jaw suspensions: amphistyly, orbitostyly, hyostyly, and euhyostyly. In amphistyly, the palatoquadrate has a postorbital articulation with the chondrocranium from which ligaments primarily suspend it anteriorly. The hyoid articulates with the mandibular arch posteriorly, but it appears to provide little support to the upper and lower jaws. In orbitostyly, the orbital process hinges with the orbital wall and the hyoid provides the majority of suspensory support.
In contrast, hyostyly involves an ethmoid articulation between the upper jaw and the cranium, while the hyoid most likely provides vastly more jaw support compared to the anterior ligaments. Finally, in euhyostyly, also known as true hyostyly, the mandibular cartilages lack a ligamentous connection to the cranium. Instead, the hyomandibular cartilages provide the only means of jaw support, while the ceratohyal and basihyal elements articulate with the lower jaw, but are disconnected from the rest of the hyoid.[9][10][11] The eyes have a tapetum lucidum. The inner margin of each pelvic fin in the male fish is grooved to constitute a clasper for the transmission of sperm. These fish are widely distributed in tropical and temperate waters.[12]
Many fish maintain buoyancy with swim bladders. However elasmobranchs lack swim bladders, and maintain buoyancy instead with large livers that are full of oil.[13] This stored oil may also function as a nutrient when food is scarce.[7][14] Deep sea sharks are usually targeted for their oil, because the livers of these species can weigh up to 20% of their total weight.[2]
Evolution
Fossilised shark teeth are known from the early Devonian, around 400 million years ago. During the following Carboniferous period, the sharks underwent a period of diversification, with many new forms evolving. Many of these became extinct during the Permian, but the remaining sharks underwent a second burst of adaptive radiation during the Jurassic, around which time the skates and rays first appeared. Many surviving orders of elasmobranch date back to the Cretaceous, or earlier.[15]
Habitats
Elasmobranchs are mostly a marine taxon, but we know several species that live in freshwater environment (approximately 60 species which represent only 5% of the 1154 described species). They can be divided into two groups.
The euryhaline elasmobranchs, which are marine species that may survive and reproduce in freshwater environment, and the obligated freshwater elasmobranchs. The second group contains elasmobranchs that only lives in freshwater environment their entire life. This group contains only one clade: the subfamily Potamotrygoninae. This clade is endemic to one specific region (which means that they can only be seen in those regions): tropical, subtropical water and wetland of South America.
Recent researches in Paraná river[16] have shown that obligated freshwater elasmobranchs were more susceptible to anthropogenic threats as overfishing and destruction of habitats due to the very small place they live in compared to the marine species.
New research has highlighted the importance of coastal wetlands, like mangroves and seagrasses, as habitats for many species of elasmobranch[17]
Taxonomy
Compagno's 2005 Sharks of the World arranges the class as follows:
- Subclass Elasmobranchii
- †Plesioselachus
- †Order Squatinactiformes
- †Order Protacrodontiformes
- †Infraclass Cladoselachimorpha
- †Order Cladoselachiformes
- †Infraclass Xenacanthimorpha
- †Order Xenacanthiformes
- Infraclass Euselachii (sharks and rays)
- †Order Ctenacanthiformes
- †Division Hybodonta
- †Order Hybodontiformes
- Division Neoselachii
- Subdivision Selachii (Selachimorpha) (modern sharks)
- Superorder Galeomorphii
- Order Heterodontiformes (bullhead sharks)
- Order Orectolobiformes (carpet sharks)
- Order Lamniformes (mackerel sharks)
- Order Carcharhiniformes (ground sharks)
- Superorder Squalomorphii
- Order Hexanchiformes (frilled and cow sharks)
- Order Squaliformes (dogfish sharks)
- †Order Protospinaciformes
- Order Squatiniformes (angel sharks)
- Order Pristiophoriformes (sawsharks)
- Superorder Galeomorphii
- Subdivision Batoidea (rays, skates, and sawfish)
- Order Torpediniformes (electric rays)
- Order Pristiformes (sawfishes)
- Order Rajiformes (skates and relatives)
- Order Myliobatiformes (stingrays and relatives)
- Subdivision Selachii (Selachimorpha) (modern sharks)
Recent molecular studies suggest the Batoidea are not derived selachians as previously thought. Instead, skates and rays are a monophyletic superorder within Elasmobranchii that shares a common ancestor with the selachians.[18][19]
See also
- Cartilaginous versus bony fishes
- List of Elasmobranch cestodes, tape worms which infect sharks, rays and skates
References
- Märss, Tiiu; Gagnier, Pierre-Yves (2001). "A new chondrichthyan from the Wenlock, Lower Silurian, of Baillie-Hamilton Island, the Canadian Arctic". Journal of Vertebrate Paleontology. 21 (4): 693–701. doi:10.1671/0272-4634(2001)021[0693:ANCFTW]2.0.CO;2.
- Vannuccini, Stefania (2002) Shark liver oil products Archived 2013-06-26 at the Wayback Machine In: Shark Utilization, Marketing and Trade, Fisheries Technical paper 389, FAO, Rome. ISBN 92-5-104361-2.
- Fowler, S.L. (2005). "Cetorhinus maximus". IUCN Red List of Threatened Species. 2005: e.T4292A10763893. doi:10.2305/IUCN.UK.2005.RLTS.T4292A10763893.en.
- "Galeorhinus galeus (School shark)". IUCN Red List of Threatened Species. 2005-06-17. 2005-06-17. Retrieved 2013-03-26.
- Guallart; et al. (2006). "Centrophorus granulosus". IUCN Red List of Threatened Species. 2006. Retrieved 11 May 2006.CS1 maint: ref=harv (link)
- Benton, Michael J. (2015). Vertebrate Palaeontology (3rd ed.). Blackwell. p. 185. ISBN 9781118406847. OCLC 945675149.
- Hoenig, J.M. and Gruber, S.H. (1990) "Life-history patterns in the elasmobranchs: implications for fisheries management" Archived 2013-02-18 at the Wayback Machine In: Elasmobranchs as living resources: advances in the biology, ecology, systematics and the status of the fisheries, eds. J. H. L. Pratt, S. H. Gruber and T. Taniuchi, US Department of Commerce, NOAA technical report NMFS 90, pp.1–16.
- "Elasmobranchii". Merriam-Webster Dictionary.
- Wilga, C.D. (2005). "Morphology and evolution of the jaw suspension in lamniform sharks". Journal of Morphology. 265 (1): 102–19. doi:10.1002/jmor.10342. PMID 15880740.
- Wilga, C. D.; Motta, P. J.; Sanford, C. P. (2007). "Evolution and ecology of feeding in elasmobranchs". Integrative and Comparative Biology. 47 (1): 55–69. doi:10.1093/icb/icm029. PMID 21672820.
- Wilga, Cheryl A.D. (2008). "Evolutionary divergence in the feeding mechanism of fishes". Acta Geologica Polonica. 58 (2): 113–20. Archived from the original on 2018-08-19. Retrieved 2017-05-24.
- Bigelow, Henry B.; Schroeder, William C. (1948). Fishes of the Western North Atlantic. Sears Foundation for Marine Research, Yale University. pp. 64–65. ASIN B000J0D9X6.
- Oguri, M (1990) "A review of selected physiological characteristics unique to elasmobranchs" Archived 2013-02-18 at the Wayback Machine In: Elasmobranchs as living resources: advances in the biology, ecology, systematics and the status of the fisheries, eds. J. H. L. Pratt, S. H. Gruber and T. Taniuchi, US Department of Commerce, NOAA technical report NMFS 90, pp.49–54.
- Bone, Q.; Roberts, B. L. (2009). "The density of elasmobranchs". Journal of the Marine Biological Association of the United Kingdom. 49 (4): 913. doi:10.1017/S0025315400038017.
- Palmer, D., ed. (1999). The Marshall Illustrated Encyclopedia of Dinosaurs and Prehistoric Animals. London: Marshall Editions. p. 26. ISBN 978-1-84028-152-1.
- Lucifora, Luis O.; Balboni, Leandro; Scarabotti, Pablo A.; Alonso, Francisco A.; Sabadin, David E.; Solari, Agustín; Vargas, Facundo; Barbini, Santiago A.; Mabragaña, Ezequiel; Díaz De Astarloa, Juan M. (2017). "Decline or stability of obligate freshwater elasmobranchs following high fishing pressure" (PDF). Biological Conservation. 210: 293–298. doi:10.1016/j.biocon.2017.04.028.
- Sievers, Michael; Brown, Christopher J.; Tulloch, Vivitskaia J.D.; Pearson, Ryan M.; Haig, Jodie A.; Turschwell, Mischa P.; Connolly, Rod M. (May 2019). "The Role of Vegetated Coastal Wetlands for Marine Megafauna Conservation". Trends in Ecology & Evolution. 34: 807–817. doi:10.1016/j.tree.2019.04.004. PMID 31126633.
- Winchell, Christopher J; Martin, Andrew P; Mallatt, Jon (2004). "Phylogeny of elasmobranchs based on LSU and SSU ribosomal RNA genes". Molecular Phylogenetics and Evolution. 31 (1): 214–24. doi:10.1016/j.ympev.2003.07.010. PMID 15019621.
- Douady, Christophe J.; Dosay, Miné; Shivji, Mahmood S.; Stanhope, Michael J. (2003). "Molecular phylogenetic evidence refuting the hypothesis of Batoidea (rays and skates) as derived sharks". Molecular Phylogenetics and Evolution. 26 (2): 215–21. doi:10.1016/S1055-7903(02)00333-0. PMID 12565032.
External links
- Skaphandrus.com Elasmobranchii