Turtle shell
The turtle shell is a highly complicated shield for the ventral and dorsal parts of turtles, tortoises and terrapins (all classified as "turtles" by zoologists), completely enclosing all the vital organs of the turtle and in some cases even the head.[1] It is constructed of modified bony elements such as the ribs, parts of the pelvis and other bones found in most reptiles. The bone of the shell consists of both skeletal and dermal bone, showing that the complete enclosure of the shell probably evolved by including dermal armor into the rib cage.

The shell of the turtle is an important study, not just because of the obvious protection it provides for the animal, but also as an identification tool, in particular with fossils as the shell is one of the likely parts of a turtle to survive fossilization. Hence understanding the structure of the shell in living species gives us comparable material with fossils.
The shell of the hawksbill turtle, among other species, has been used as a material for a wide range of small decorative and practical items since antiquity, but is normally referred to as tortoiseshell.
Shell nomenclature
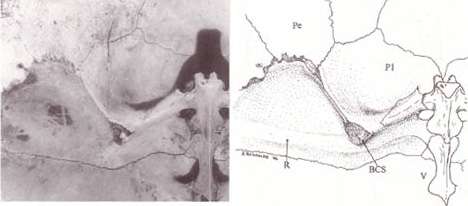
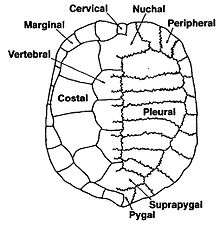
The turtle shell is made up of numerous bony elements, generally named after similar bones in other vertebrates, and a series of keratinous scutes which are also uniquely named. Some of those bones that make the top of the shell, carapace, evolved from the scapula rami of the clavicles along with the dorsal and superficial migration of the cleithra.[2] The ventral surface is called the plastron.[3][4] These are joined by an area called the bridge. The actual suture between the bridge and the plastron is called the anterior bridge strut.[5] In Pleurodires the posterior pelvis is also part of the carapace, fully fused with it. This is not the case in Cryptodires which have a floating pelvis.[3][4] The anterior bridge strut and posterior bridge strut are part of the plastron, on the carapace are the sutures into which they insert, known as the Bridge carapace suture.[5]
The bones of the shell are named for standard vertebrate elements. As such the carapace is made up of 8 pleurals on each side, these are a combination of the ribs and fused dermal bone. Outside of this at the anterior of the shell is the single nuchal bone, a series of 12 paired periphals then extend along each side. At the posterior of the shell is the pygal bone and in front of this nested behind the eighth pleurals is the suprapygal.[3]
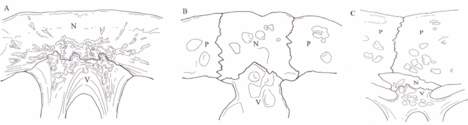
Between each of the pleurals are a series of neural bones,[6] which although always present are not always visible,[7] in many species of Pleurodire they are submerged below the pleurals.[8] Beneath the neural bone is the Neural arch which forms the upper half of the encasement for the spinal chord. Below this the rest of the vertebral column.[4] Some species of turtles have some extra bones called mesoplastra, which are located between the carapace and plastron in the bridge area. They are present in most Pelomedusid turtles.[9]
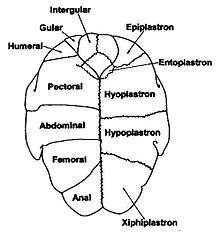
The skeletal elements of the plastron are also largely in pairs. Anteriorly there are two epiplastra, with the hyoplastra behind them. These enclose the singuar entoplastron. These make up the front half of the plastron and the hyoplastron contains the anterior bridge strut. The posterior half is made up of two hypoplastra (containing the posterior bridge strut) and the rear is a pair of xiphiplastra.[4][5]
Overlying the boney elements are a series of scutes, which are made of keratin and are a lot like horn or nail tissue. In the center of the carapace are 5 vertebral scutes and out from these are 4 pairs of costal scutes. Around the edge of the shell are 12 pairs of marginal scutes. All these scutes are aligned so that for the most part the sutures between the bones are in the middle of the scutes above. At the anterior of the shell there may be a cervical scute (sometimes incorrectly called a nuchal scute) however the presence or absence of this scute is highly variable, even within species.[4][9]
On the plastron there are two gular scutes at the front, followed by a pair of pectorals, then abdominals, femorals and lastly anals. A particular variation is the Pleurodiran turtles have an intergular scute between the gulars at the front, giving them a total of 13 plastral scutes. Compared to the 12 in all Cryptodiran turtles.[4][9]
Carapace
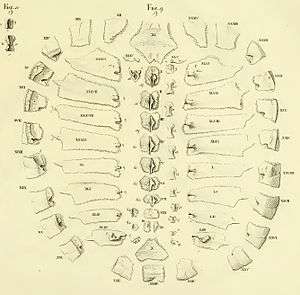
The carapace is the dorsal (back), convex part of the shell structure of a turtle, consisting of the animal's ossified ribs fused with the dermal bone. The spine and expanded ribs are fused through ossification to dermal plates beneath the skin to form a hard shell. Exterior to the skin the shell is covered by scutes, which are horny plates made of keratin that protect the shell from scrapes and bruises. A keel, a ridge that runs from front to the back of the animal is present in some species, these may be single, paired or even three rows of them. In most turtles the shell is relatively uniform in structure, species variation in general shape and color being the main differences. However the soft shell turtles, pig-nose turtles and the leatherback sea turtle have lost the scutes and reduced the ossification of the shell. This leaves the shell covered only by skin.[11] These are all highly aquatic forms.
The evolution of the turtle's shell is unique because of how the carapace represents transformed vertebrae and ribs. While other tetrapods have their scapula, or shoulder blades, found outside of the ribcage, the scapula for turtles is found inside the ribcage.[12][13] The shells of other tetrapods, such as armadillos, are not linked directly to the vertebral column or rib cage allowing the ribs to move freely with the surrounding intercostal muscle.[14] However, analysis of the transitional fossil, Eunotosaurus africanus shows that early ancestors of turtles lost that intercostal muscle usually found between the ribs.[15]
Recent breakthroughs in stem-turtle fossil records contribute to the study of the evolution of the turtle's shell. The first piece of fossil record discovered, essential for building the evolution and development model, was Germany and Thailand's 214-million-year-old Late Triassic reptile Proganochelys, which marked as the first point of full shell development and carapace ossification in Testudines.[16]
The following phenomenal discovery of China's 220-million-year-old stem turtle, predating the Proganonchelys by 6 million years, the Odontochelys semitestacea fossil in China shed light on the intermediate stages of turtle carapace evolution by exhibiting a partially formed dorsal carapace.[17] One major discovery was that O. semitestacea provided documentation that the plastron evolved before the carapace structure.[18] Close examination of the partially formed carapace revealed similarity to the fully formed carapace in crown turtles such as the lack of intercostal muscles and limited rib mobility. Furthermore, it is made up of modified forms of laterally expanded and broadened ribs without ossification, similar in structure to the modern turtle embryo.[16]
The addition of South Africa's 260-million-year-old Permian stem reptile Eunotosaurus africanus, the hypothesized earliest stem turtle predating the O. semitestacea by 40 million years, revealed the early stages of carapace evolution. Histological data characterized E. africanus and Proganochelys ribs as homologous structures, both broadened and T-shaped in cross-section.[19]
Lyson hypothesizes that this morphology in Eunotosaurus africanus suggests that turtles may have fossorial origin. The wide torso gave rise to the turtle shell but during the Permian period, the broadened ribs may have provided great stability in burrowing. The skeletal structure of E. africanus in comparison to the extant fossorial gopher tortoise share similar features adapted to withstand the impact and force needed in digging. For example, E. africanus exhibits shoulders and forelimb adapted to burrowing, showing increased muscle indicated in structures such as their tubercle on the posterior coracoid and their large and wide terminal phalanges. Furthermore, fossoriality may have helped E. africanus survive the global mass extinction which wiped out over ninety percent of species at the end of the Permian period.[20][21] This feature is also found in a stem of crown turtles, introducing the possibility that fossorial behavior was not subject to E. africanus , but rather played an essential role in the early evolution of shelled turtles. [20]
The most recent finding of a Middle Triassic stem-turtle offers both a morphological and temporal intermediate to the Eunotosaurus africanus and Odontochelys semitestacea, contributing to the construction of carapace evolution timeline. The 240 million-year-old Pappochelys fossil found in Germany shows similarly broadened and T-shaped ribs that vary in shape with respect to position along the spine.[22]
The carapacial ridge has been found to play an essential role in the development of the turtle shell. Embryological analyses show that the carapacial ridge initiates the formation of the turtle shell.[23] It causes axial arrest which causes the ribs to be dorsalized, the shoulder girdle to be rearranged and encapsulated in the rib cage, and the carapace to develop.[24] Odontochelys semitestacea presents evidence of axial arrest that is observed in embryos but lacks fan-shaped ribs and a carapace. This suggests that the primitive carapacial ridge functioned differently and must have gained the function of mediating the ribs and carapace development later.[17][25] The Pax1 and Sonic hedgehog gene (Shh) serve as key regulators during the development of the vertebral column. Shh expression in the neural tube is essential for the maintenance of Pax1 expression in the ventral sclerotome and thus plays a key role in carapacial rib development. Genetic observations of Pax1 and Shh further provide an understanding in key gene expression that could potentially be responsible for changing turtle morphology. [26]
Plastron


The plastron (plural: plastrons or plastra) is the nearly flat part of the shell structure of a turtle, what one would call the belly or ventral surface of the shell. It also includes within its structure the anterior and posterior bridge struts and the bridge of the shell.[4][5] The plastron is made up of nine bones and the two epiplastra at the anterior border of the plastron are homologous to the clavicles of other tetrapods.[27] The rest of the plastral bones are homologous to the gastralia of other tetrapods. The plastron has been described as an exoskeleton, like osteoderms of other reptilians; but unlike osteoderms, the plastron also possesses osteoblasts, the osteoid, and the periosteum.[28]
The evolution of the plastron has remained more mysterious, though Georges Cuvier, a French naturalist and zoologist in the 19th century, wrote that the plastron developed primarily from the sternum of the turtle.[29] This fits well with the knowledge obtained through embryological studies, showing that changes in the pathways of rib development often result in malformation or loss of the plastron. This phenomenon occurs in turtle development, but instead of experiencing complete loss of the sternum the turtle body plan repurposes the bone into the form of the plastron,[25] although other analyses find that the endochondral sternum is absent and replaced by the exoskeletal plastron. The ventral ribs are effectively not present, replaced by the plastron, unless the gastralia from which the plastron evolved were once floating ventral ribs.[28] During turtle evolution, there was probably a division of labor between the ribs, which specialized to stabilize the trunk, and the abdominal muscles, which specialized for respiration, and these changes took place 50 million years before the shell was fully ossified.[30]
The discovery of an ancestral turtle fossil, Pappochelys rosinae, provides additional clues as to how the plastron formed. Pappochelys serves as an intermediate form between two early stem-turtles, E. africanus and Odontochelys, the latter of which possesses a fully formed plastron. In place of a modern plastron, Pappochelys has paired gastralia, like those found in E. africanus. Pappochelys is different from its ancestor because the gastralia show signs of having once been fused, as indicated by the fossil specimens which show forked ends. This evidence shows a gradual change from paired gastralia, to paired and fused gastralia, and finally to the modern plastron across these three specimens.[22]
In certain families there is a hinge between the pectoral and abdominal scutes allowing the turtle to almost completely enclose itself. In certain species the sex of a testudine can be told by whether the plastron is concave, male or convex, female. This is because of the mating position; the male's concave plastron allows it to more easily mount the female during copulation.
The plastral scutes join along a central seam down the middle of the plastron. The relative lengths of the seam segments can be used to help identify a species of turtle. There are six laterally symmetric pairs of scutes on the plastron: gular, humeral, pectoral, abdominal, femoral, and anal (going from the head to the tail down the seam); the abdominal and gular scute seams are approximately the same length, and the femoral and pectoral seams are approximately the same length.
The gular scute or gular projection on a turtle is the most anterior part of the plastron, the underside of the shell. Some tortoises have paired gular scutes, while others have a single undivided gular scute. The gular scutes may be referred to as a gular projection if they stick out like a trowel.


- Gular (disambiguation), gular anatomical formations in other species
Plastral formula
The plastral formula is used to compare the sizes of the individual plastral scutes (measured along the midseam). The following plastral scutes are often distinguished (with their abbreviation):
intergular = intergul gular = gul humeral = hum pectoral = pect abdominal = abd femoral = fem anal = an
Comparison of the plastral formulas provides distinction between the two species. For example, for the eastern box turtle, the plastral formula is: an > abd > gul > pect > hum >< fem[31]
Turtle plastrons were used by the ancient Chinese in a type of divination called plastromancy. See also Oracle bones.
Origin
Studies of two ancestral turtles, the polka dot turtle ancestor[32] and the Late Triassic turtle Proganochelys[33] have postulated the ancestral form of the carapace. It was hypothesized that the carapace was originated from scattered osteoderms that cover an incompletely developed outer shell. The scattered osteoderms would first fuse with each other and then fuse with the underlying endoskeletal vertebrae and ribs in evolution. This osteoderm pattern embedded in the skin is also shown in many other reptiles. A gradual transformation of the turtle shell from ancestral forms to modern ones was proposed by Michael S. Y. Lee:[34] The transformation series of the carapace first start with some unarmored parareptile, then armored pareiasaur, and finally develop into modern turtles with fully developed carapace and relocated rib cage. This transformationalist perspective of the turtle shell origin is consistent with the Darwinian view, which agrees on the theory that descendant structures are derived from gradual, stepwise modification of ancestral ones.
The discovery of the ancestral turtle fossil Eunotosaurus africanus (260-million-year-old) provided more evidence for the diapsid origin of the turtles. The Eunotosaurus africanus was described as "a diapsid reptile in the process of becoming secondarily anapsid",[35] thus filling a crucial gap in the evolutionary transformation of turtle from diapsid ancestor reptiles to anapsid modern turtles. The phylogenetic relationship of the ancestral turtles is summarized as the follows: "the Eunotosaurus is placed at the bottom of the stem section of the turtle tree, followed by Pappochelys and Odontochelys along the turtle stem and on to more crown-ward turtles".[36]
Scutes

The turtle's shell is covered in scutes that are made of keratin. The individual scutes as shown above have specific names and are generally consistent across the various species of turtles. Terrestrial tortoises do not shed their scutes. New scutes grow by the addition of keratin layers to the base of each scute. Aquatic chelonii shed individual scutes. The scute effectively forms the skin over the underlying bony structures; there is a very thin layer of subcutaneous tissue between the scute and the skeleton. The scutes can be brightly colored in some species, but the basal color is a grey to dark brown color dorsally; the plastral scutes are often white to yellow in base color. Moustakas-Verho and Cherepanov's embryological study reveals that the patterning of the plastral scutes appear independent from the patterning of carapacial scutes, suggesting that the carapace and plastron evolved separately.[37]
The appearance of scutes correlates to the transition from aquatic to terrestrial mode of life in tetrapods during the Carboniferous period (340 Ma).[38] In the evolution from amphibians to terrestrial amniotes, transition in a wide variety of skin structures occurred. Ancestors of turtles likely diverged from amphibians to develop a horny cover in their early terrestrial ancestral forms.[39]
Diseases

Septicemic cutaneous ulcerative disease (SCUD) / shell rot
In septicemic cutaneous ulcerative disease (SCUD) or "shell rot", originally described by Kaplan (1957),[40] ulcers of the shell may be superficial or deep. Ulcers may be seen on both the shell and legs. The disease is known to be caused by a variety of bacteria or fungi entering through some sort of abrasion, combined with poor animal husbandry. The disease is identified by its progression and what starts as ulcerative lesions of the plastron leads to a septicemic infection causing the degradation of the liver and other organs.[41] Without treatment, this will lead to death. The condition is often associated with the bacteria, Citrobacter freundii.
Turtles with ulcerative shell lesions should be examined and treated by a veterinarian, as the ulcers may become infected and penetrate through the shell. The shell will need to be cleaned daily, and dead tissue removed. Topical and/or injectable antibiotics are required in the case of bacterial infections. Deep ulcers may need to be repaired through surgery and the application of acrylic or fiberglass material.
In remote areas where no specialised vets are available some success has been achieved in treating shell rot by carefully removing dead tissue, disinfecting the areas with hydrogen peroxide and then treating the affected areas with propolis dissolved in alcohol where the shell is affected or applying propolis salve where soft tissue is affected.[42][43]
Pyramiding

Pyramiding is a shell deformity sometimes found in captive tortoises, in which the shell grows unevenly resulting in a pyramid shape underlying each scute. This deformity can vary in severity from barely noticeable to life-threatening. Indian star tortoises and some other species are more prone to this condition than others.
Several factors may exacerbate pyramiding, however the condition is strongly linked to the availability of moisture to facilitate the proper distribution of keratin growth which makes up the shells of tortoises. If a tortoise is dehydrated or unable to access conditions which are sufficiently moist, the keratinous layers which would otherwise form at the edges of scutes grow beneath the exisiting hardened shell causing a stacking effect which pushes shell growth upwards rather than outwards and exerts pressure on the skeleton beneath the shell. If severe, this leads to spinal and physical malformation.
Other factors which may also contribute to pyramiding include the consumption of excessive animal or vegetable protein; inadequate calcium, UVB and/or vitamin D3; poor nutrition.[44][45][46] Pyramiding may also be a visible sign of metabolic bone disease (MBD) in tortoises. Once pyramiding has occurred, it cannot be reversed, though if the underlying problems are corrected, any subsequent shell growth will form smoothly.
Broken shells
Turtles' shells may become broken due to natural causes, accidents, or intention.[47] When the split is not too wide the shell may be brought together by screwing bolts into the shell then connecting the bolts with a wire; otherwise, a special device may be required.[48]
Turtleshell masks
Torres Strait Islander people are the only culture in the world to make turtleshell masks, known as krar (turtleshell) in the Western Islands and le-op (human face) in the Eastern Islands.[49]

See also
References
- Cordero, GA (2017). "The Turtle's Shell". Current Biology. 27 (5): R168–R169. doi:10.1016/j.cub.2016.12.040. PMID 28267966.
- Lyson, Tyler R.; Bhullar, Bhart-Anjan S.; Bever, Gabe S.; Joyce, Walter G.; de Queiroz, Kevin; Abzhanov, Arhat; Gauthier, Jacques A. (2013-09-01). "Homology of the enigmatic nuchal bone reveals novel reorganization of the shoulder girdle in the evolution of the turtle shell". Evolution & Development. 15 (5): 317–325. doi:10.1111/ede.12041. ISSN 1525-142X. PMID 24074278.
- Romer, A.S. (1956) Osteology of the Reptiles. Univ. of Chicago Press.
- Zangerl, R. 1969. The turtle shell. In: Gans, C., Bellairs, D.d'A. and Parsons, T.A. (Eds). Biology of the Reptilia, Vol 1, Morphology A. London: Academic Press. pp. 311–340
- Thomson, S., White, A. & Georges, A (1997). "Re-Evaluation of Emydura lavarackorum: Identification of a Living Fossil" (PDF). Memoirs of the Queensland Museum. 42 (1): 327–336. Archived from the original (PDF) on 2015-06-09.CS1 maint: multiple names: authors list (link)
- Pritchard, P.C.H. (1988). "A survey of neural bone variation among recent chelonian species, with functional interpretations". Acta Zoologica Cracoviensa. 31 (26): 625–686.
- Thomson, S. & Georges, A. (1996). "Neural bones in chelid turtles". Chelonian Conservation and Biology. 2: 82–86.
- Rhodin, A.G.J. & Mittermeier, R.A. (1977). "Neural bones in chelid turtles from Australia and New Guinea" (PDF). Copeia. 1977 (2): 370–372. doi:10.2307/1443917. JSTOR 1443917.
- Pritchard, P.C.H., and P. Trebbau. 1984. The Turtles of Venezuela. SSAR Contributions to Herpetology 2:.
- Bojanus, L. H. 1819. Anatome testudinis Europaeae. 178pp, 31 plates
- Chen, I. H., W. Yang, and M. A. Meyers (2015). "Leatherback sea turtle shell: A tough and flexible biological design". Acta Biomaterialia. 28: 2–12. doi:10.1016/j.actbio.2015.09.023. PMID 26391496.CS1 maint: multiple names: authors list (link)
- Nagashima, H.; Sugahara, F.; Takechi, M.; Ericsson, R.; Kawashima-Ohya, Y.; Narita, Y.; Kuratani, S. (2009). "Evolution of the turtle body plan by the folding and creation of new muscle connections". Science. 325 (5937): 193–196. Bibcode:2009Sci...325..193N. doi:10.1126/science.1173826. PMID 19590000.
- Wang, Z., J. Pascual-Anaya, A. Zadissa, W. Q. Li, Y. Niimura, Z. Y. Huang, C. Y. Li et al. 2013. The draft genomes of soft-shell turtle and green sea turtle yield insights into the development and evolution of the turtle-specific body plan" Nature Genetics 45:701-+.
- Hirasawa, T., H. Nagashima, and S. Kuratani. 2013. The endoskeletal origin of the turtle carapace. Nature Communications 4.
- Lee, M. S. Y. (2013). "Palaeontology: Turtles in transition". Current Biology. 23 (12): R513–R515. doi:10.1016/j.cub.2013.05.011. PMID 23787042.
- Li, C.; Wu, X.-C.; Rieppel, O.; Wang, L.-T.; Zhao, L.-J. (2008). "An ancestral turtle from the Late Triassic of southwestern China". Nature. 456 (7221): 497–501. Bibcode:2008Natur.456..497L. doi:10.1038/nature07533. PMID 19037315.
- Kuratani, S (2011). "Evolutionary developmental perspective for the origin of turtles: the folding theory for the shell based on the developmental nature of the carapacial ridge". Evolution & Development. 13 (1): 1–14. doi:10.1111/j.1525-142x.2010.00451.x. PMID 21210938.
- Li, Chun; Wu, Xiao-Chun; Rieppel, Olivier; Wang, Li-Ting; Zhao, Li-Jun (2008-11-27). "An ancestral turtle from the Late Triassic of southwestern China". Nature. 456 (7221): 497–501. Bibcode:2008Natur.456..497L. doi:10.1038/nature07533. ISSN 0028-0836. PMID 19037315.
- Lyson, Tyler R.; Bever, Gabe S.; Scheyer, Torsten M.; Hsiang, Allison Y.; Gauthier, Jacques A. (2013). "Evolutionary Origin of the Turtle Shell". Current Biology. 23 (12): 1113–1119. doi:10.1016/j.cub.2013.05.003. PMID 23727095.
- Lyson, T. R., B. S. Rubidge, T. M. Scheyer, K. De Queiroz, E. R. Schachner, R. M. Smith, J. Botha-Brink; et al. (2016). "Fossorial Origin of the Turtle Shell" (PDF). Current Biology. 26 (14): 1887–1894. doi:10.1016/j.cub.2016.05.020. PMID 27426515.CS1 maint: multiple names: authors list (link)
- Chen, Z.-Q., and M. J. Benton. (2012). "The timing and pattern of biotic recovery following the end-Permian mass extinction". Nature Geoscience. 5 (6): 375–383. Bibcode:2012NatGe...5..375C. doi:10.1038/ngeo1475.CS1 maint: multiple names: authors list (link)
- Schoch, Rainer R.; Sues, Hans-Dieter (2015). "A Middle Triassic stem-turtle and the evolution of the turtle body plan". Nature. 523 (7562): 584–587. Bibcode:2015Natur.523..584S. doi:10.1038/nature14472. PMID 26106865.
- Ruckes, Herbert (December 1929). "STUDIES IN CHELONIAN OSTEOLOGY, Part I: TRUSS AND ARCH ANALOGIES IN CHELONIAN PELVES". Annals of the New York Academy of Sciences. 31 (1): 31–80. doi:10.1111/j.1749-6632.1929.tb55191.x. ISSN 0077-8923.
- Rieppel, Olivier (2017). Turtles as hopeful monsters : origins and evolution. Indiana University Press. p. 146. ISBN 9780253024756. OCLC 1037017014.
- Hirasawa, Tatsuya; Pascual-Anaya, Juan; Kamezaki, Naoki; Taniguchi, Mari; Mine, Kanako; Kuratani, Shigeru (2015-05-01). "The evolutionary origin of the turtle shell and its dependence on the axial arrest of the embryonic rib cage". Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 324 (3): 194–207. doi:10.1002/jez.b.22579. ISSN 1552-5015. PMID 24898540.
- Moustakas-Verho, Jacqueline; Thomas, C. T.; Gilbert, S. F. (2017). "Patterning of the turtle shell". Current Opinion in Genetics and Development. 45: 124–131. doi:10.1016/j.gde.2017.03.016. PMID 28570929 – via Elsevier Science Direct.
- Gilbert, S. F.; Loredo, G. A.; Brukman, A.; Burke, A. C. (2001). "Morphogenesis of the turtle shell: The development of a novel structure in tetrapod evolution". Evolution & Development. 3 (2): 47–58. doi:10.1046/j.1525-142x.2001.003002047.x. PMID 11341674.
- Rice, Ritva; Kallonen, Aki; Cebra-Thomas, Judith; Gilbert, Scott F. (2016-05-10). "Development of the turtle plastron, the order-defining skeletal structure". Proceedings of the National Academy of Sciences. 113 (19): 5317–5322. doi:10.1073/pnas.1600958113. ISSN 0027-8424. PMC 4868452. PMID 27114549.
- MacCord, Kate; Caniglia, Guido; Moustakas-Verho, Jacqueline E.; Burke, Ann C. (2015-05-01). "The dawn of chelonian research: Turtles between comparative anatomy and embryology in the 19th century". Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 324 (3): 169–180. doi:10.1002/jez.b.22587. hdl:10138/223805. ISSN 1552-5015. PMID 25074288.
- Lyson, Tyler R.; Schachner, Emma R.; Botha-Brink, Jennifer; Scheyer, Torsten M.; Lambertz, Markus; Bever, G. S.; Rubidge, Bruce S.; de Queiroz, Kevin (2014-11-07). "Origin of the unique ventilatory apparatus of turtles". Nature Communications. 5 (1): 5211. Bibcode:2014NatCo...5.5211L. doi:10.1038/ncomms6211. ISSN 2041-1723. PMID 25376734.
- C.H. Ernst, R.G.M. Altenburg & R.W. Barbour. "Terrapene carolina". Netherlands Biodiversity Information Facility. Archived from the original on 24 July 2011. Retrieved 12 February 2011.
- Rieppel, Olivier (2017-03-13). Turtles as hopeful monsters: origins and evolution. Bloomington, Indiana. p. 157. ISBN 9780253025074. OCLC 962141060.
- Gaffney, Eugene S. (1990). The comparative osteology of the Triassic turtle Proganochelys. OCLC 263164288.
- Lee, Michael S. Y. (February 1996). "Correlated progression and the origin of turtles". Nature. 379 (6568): 812–815. doi:10.1038/379812a0. ISSN 0028-0836.
- Bever, G. S.; Lyson, Tyler R.; Field, Daniel J.; Bhullar, Bhart-Anjan S. (September 2015). "Evolutionary origin of the turtle skull". Nature. 525 (7568): 239–242. doi:10.1038/nature14900. ISSN 0028-0836. PMID 26331544.
- Rieppel, Olivier (2017). Turtles as hopeful monsters : origins and evolution. Indiana University Press. p. 70. ISBN 9780253024756. OCLC 1037017014.
- Moustakas-Verho, J. E., R. Zimm, J. Cebra-Thomas, N. K. Lempiainen, A. Kallonen, K. L. Mitchell, K. Hamalainen; et al. (2014). "The origin and loss of periodic patterning in the turtle shell" (PDF). Development. 141 (15): 3033–3039. doi:10.1242/dev.109041. PMID 25053434.CS1 maint: multiple names: authors list (link)
- Zimm, R.; Wyneken, Bentley, J. (2017). "Environmental causation of turtle scutes anomalies". Integrative and Comparative Biology. 57 (6): 1303–1311. doi:10.1093/icb/icx066. PMID 28992039 – via Society for Integrative and Comparative Biology.
- Cherepanov, G. O. (2015). "Scute's polymorphism as a source of evolutionary development of the turtle shell". Paleontological Journal. 49 (14): 1635–1644. doi:10.1134/S003103011514004X – via St. Petersburg State University.
- Kaplan, H. M. (1957). "Septicemic, cutaneous ulcerative disease of turtles". Proc. Animal Care Panel. 7: 273–277.
- Mader, D. (2006) Reptile Medicine and Surgery, 2nd ed., Saunders, ISBN 072169327X.
- B Pitzer 2018, personal communication, 25 April.
- R Ilek 2019, personal communication, 1 June).
- Gerlach, J (2004). "Effects of diet on the systematic utility of the tortoise carapace" (PDF). Island Biodiversity. Retrieved 17 July 2019.
- Innis, Charles. "BASIC HUSBANDRY AND NUTRITION OF CHELONIANS" (PDF). Cabi.Org.
- "Pyramiding in Tortoises". www.reptilesmagazine.com.
- Lee Moran (June 13, 2013). "Wisconsin's Delbrook Golf Club golfers beat turtle to death in sand bunker — as she tried to lay her eggs". New York Daily News.
- Viva Sarah Press (July 26, 2012). "Israeli device fixes broken turtle shell".
- "Art Sets. Art of the Torres Strait Islands". New South Wales Art Gallery. Retrieved 7 January 2020.
External links
- African Tortoise Website, has more information on pyramiding