Parental investment
Parental investment, in evolutionary biology and evolutionary psychology, is any parental expenditure (e.g. time, energy, resources) that benefits offspring.[1][2] Parental investment may be performed by both males and females (biparental care), females alone (exclusive maternal care) or males alone (exclusive paternal care). Care can be provided at any stage of the offspring's life, from pre-natal (e.g. egg guarding and incubation in birds, and placental nourishment in mammals) to post-natal (e.g. food provisioning and protection of offspring).

Parental investment theory, a term coined by Robert Trivers in 1972, predicts that the sex that invests more in its offspring will be more selective when choosing a mate, and the less-investing sex will have intra-sexual competition for access to mates. This theory has been influential in explaining sex differences in sexual selection and mate preferences, throughout the animal kingdom and in humans.[2]
History
In 1859, Charles Darwin published On the Origin of Species.[3] This introduced the concept of natural selection to the world, as well as related theories such as sexual selection. For the first time, evolutionary theory was used to explain why females are "coy" and males are "ardent" and compete with each other for females' attention. In 1930, Ronald Fisher wrote The Genetical Theory of Natural Selection,[4] in which he introduced the modern concept of parental investment, introduced the sexy son hypothesis, and introduced Fisher's principle. In 1948, Angus John Bateman published an influential study of fruit flies in which he concluded that because female gametes are more costly to produce than male gametes, the reproductive success of females was limited by the ability to produce ovum, and the reproductive success of males was limited by access to females.[5] In 1972, Trivers continued this line of thinking with his proposal of parental investment theory, which describes how parental investment affects sexual behavior. He concludes that the sex that has higher parental investment will be more selective when choosing a mate, and the sex with lower investment will compete intra-sexually for mating opportunities.[2] In 1974, Trivers extended parental investment theory to explain parent-offspring conflict, the conflict between investment that is optimal from the parent's versus the offspring's perspective.[6]
Parental care
Parental investment theory is a branch of life history theory. The earliest consideration of parental investment is given by Ronald Fisher in his 1930 book The Genetical Theory of Natural Selection,[7] wherein Fisher argued that parental expenditure on both sexes of offspring should be equal. Clutton-Brock expanded the concept of parental investment to include costs to any other component of parental fitness.
Male dunnocks tend to not discriminate between their own young and those of another male in polyandrous or polygynandrous systems. They increase their own reproductive success through feeding the offspring in relation to their own access to the female throughout the mating period, which is generally a good predictor of paternity.[8] This indiscriminative parental care by males is also observed in redlip blennies.[9]

In some insects, male parental investment is given in the form of a nuptial gift. For instance, ornate moth females receive a spermatophore containing nutrients, sperm and defensive toxins from the male during copulation. This gift, which can account for up to 10% of the male's body mass, constitutes the total parental investment the male provides.[10]
In some species, such as humans and many birds, the offspring are altricial and unable to fend for themselves for an extended period of time after birth. In these species, males invest more in their offspring than do the male parents of precocial species, since reproductive success would otherwise suffer.
The benefits of parental investment to the offspring are large and are associated with the effects on condition, growth, survival, and ultimately on reproductive success of the offspring. For example, in the cichlid fish Tropheus moorii, a female has very high parental investment in her young because she mouthbroods the young and while mouthbrooding, all nourishment she takes in goes to feed the young and she effectively starves herself. In doing this, her young are larger, heavier, and faster than they would have been without it. These benefits are very advantageous since it lowers their risk of being eaten by predators and size is usually the determining factor in conflicts over resources.[11] However, such benefits can come at the cost of parent's ability to reproduce in the future e.g., through increased risk of injury when defending offspring against predators, loss of mating opportunities whilst rearing offspring, and an increase in the time interval until the next reproduction.
A special case of parental investment is when young do need nourishment and protection, but the genetic parents do not actually contribute in the effort to raise their own offspring. For example, in Bombus terrestris, oftentimes sterile female workers will not reproduce on their own, but will raise their mother's brood instead. This is common in social Hymenoptera due to haplodiploidy, whereby males are haploid and females are diploid. This ensures that sisters are more related to each other than they ever would be to their own offspring, incentivizing them to help raise their mother's young over their own.[12]
Overall, parents are selected to maximize the difference between the benefits and the costs, and parental care will be likely to evolve when the benefits exceed the costs.
Parent-offspring conflict
Reproduction is costly. Individuals are limited in the degree to which they can devote time and resources to producing and raising their young, and such expenditure may also be detrimental to their future condition, survival, and further reproductive output. However, such expenditure is typically beneficial to the offspring, enhancing their condition, survival, and reproductive success. These differences may lead to parent-offspring conflict. Parents are naturally selected to maximize the difference between the benefits and the costs, and parental care will tend to exist when the benefits are substantially greater than the costs.
Parents are equally related to all offspring, and so in order to optimize their fitness and chance of reproducing their genes, they should distribute their investment equally among current and future offspring. However, any single offspring is more related to themselves (they have 100% of their DNA in common with themselves) than they are to their siblings (siblings usually share 50% of their DNA), it is best for the offspring's fitness if the parent(s) invest more in them. To optimize fitness, a parent would want to invest in each offspring equally, but each offspring would want a larger share of parental investment. The parent is selected to invest in the offspring up until the point at which investing in the current offspring is costlier than investing in future offspring.[13]
In iteroparous species, where individuals may go through several reproductive bouts during their lifetime, a tradeoff may exist between investment in current offspring and future reproduction. Parents need to balance their offspring's demands against their own self-maintenance. This potential negative effect of parental care was explicitly formalized by Trivers in 1972, who originally defined the term parental investment to mean any investment by the parent in an individual offspring that increases the offspring's chance of surviving (and hence reproductive success) at the cost of the parent's ability to invest in other offspring.[2]
Penguins are a prime example of a species that drastically sacrifices their own health and well-being in exchange for the survival of their offspring. This behavior, one that does not necessarily benefit the individual, but the genetic code from which the individual arises, can be seen in the King Penguin. Although some animals do exhibit altruistic behaviors towards individuals that are not of direct relation, many of these behaviors appear mostly in parent-offspring relationships. While breeding, males remain in a fasting-period at the breeding site for five weeks, waiting for the female to return for her own incubation shift. However, during this time period, males may decide to abandon their egg if the female is delayed in her return to the breeding grounds.[14]
It shows that these penguins initially show a trade-off of their own health, in hopes of increasing the survivorship of their egg. But there comes a point where the male penguin's costs become too high in comparison to the gain of a successful breeding season. Olof Olsson investigated the correlation between how many experiences in breeding an individual has and the duration an individual will wait until abandoning his egg. He proposed that the more experienced the individual, the better that individual will be at replenishing his exhausted body reserves, allowing him to remain at the egg for a longer period of time.[14]
The males' sacrifice of their body weight and possible survivorship, in order to increase their offspring's chance of survival is a trade-off between current reproductive success and the parents' future survival.[14] This trade-off makes sense with other examples of kin-based altruism and is a clear example of the use of altruism in an attempt to increase overall fitness of an individual's genetic material at the expense of the individual's future survival.
Maternal-offspring conflict in investment
The maternal-offspring conflict has also been studied in animals species and humans. One such case has been documented in the mid-1970s by ethologist Wulf Schiefenhövel. Eipo women of West New Guinea engage in a cultural practice in which they give birth just outside the village. Following the birth of their child, each woman weighed whether or not she should keep the child or leave the child in the brush nearby, inevitably ending in the death of the child.[15] Likelihood of survival and availability of resources within the village were factors that played into this decision of whether or not to keep the baby. During one illustrated birth, the mother felt the child was too ill and would not survive, so she wrapped the child up, preparing to leave the child in the brush; however, upon seeing the child moving, the mother unwrapped the child and brought it into the village, demonstrating a shift of life and death.[15] This conflict between the mother and the child resulted in detachment behaviors in Brazil, seen in Scheper-Hughes work as "many Alto babies remain[ed] not only unchristened but unnamed until they begin to walk or talk",[16] or if a medical crisis arose and the baby needed an emergency baptism. This conflict between survival, both emotional and physical, prompted a shift in cultural practices, thus resulting in new forms of investment from the mother towards the child.
Alloparental care
Alloparental care also referred to as 'Allomothering,' is when a member of a community, apart from the biological parents of the infant, partake in offspring care provision.[17] A range of behaviors fall under the term alloparental care, some of which are: carrying, feeding, watching over, protecting, and grooming. Through alloparental care stress on parents, especially the mother, can be reduced, therefore reducing the negative effects of the parent-offspring conflict on the mother.[18] In While the apparent altruistic nature of the behavior may seem at odds with Darwin's theory of natural selection, as taking care of offspring which are not one's own would not increase one's direct fitness, while taking time, energy and resources away from raising one's own offspring, the behavior can be explained evolutionarily as increasing indirect fitness, as the offspring is likely to be non-descendent kin, therefore carrying some of the genetics of the alloparent.[17]
Offspring and situation direction
Parental investment behavior enhances the chances of survival of offspring, and it does not require underlying mechanisms to be compatible with empathy applicable to adults, or situations involving unrelated offspring, and it does not require the offspring to reciprocate the altruistic behavior in any way.[19][20] Parentally investing individuals are not more vulnerable to being exploited by other adults.
Trivers' parental investment theory
Parental investment as defined by Trivers in 1972[21] is the investment in offspring by the parent that increases the offspring's chances of surviving and hence reproductive success at the expense of the parent's ability to invest in other offspring. A large parental investment largely decreases the parents' chances of investing in other offspring. Parental investment can be split into two main categories: mating investment and rearing investment. Mating investment consist of the sexual act and the sex cells invested. The rearing investment is the time and energy expended to raise the offspring after conception. Women's parental investment in both mating and rearing efforts greatly surpasses that of the male. In terms of sex cells (egg and sperms cells), the female's investment is a lot larger, while males produce thousands of sperm cells which are supplied at a rate of twelve million per hour.[22]
Women have a fixed supply of around 400 ova. Also, fertilization and gestation occur in women, investments which outweigh the male's investment of just one sperm cell. Furthermore, for women, one act of sexual intercourse could result in a nine-month commitment such as human gestation and subsequent commitments related to rearing such as breastfeeding. From Trivers' theory of parental investment, several implications follow. The first implication is that women are often but not always the more investing sex. The fact that they are often the more investing sex leads to the second implication that evolution favors females who are more selective of their mates to ensure that intercourse would not result in unnecessary or wasteful costs. The third implication is that because women invest more and are essential for the reproductive success of their offspring, they are a valuable resource for men; as a result, males often compete for sexual access to females.
Males as the more investing sex
For many species the only type of male investment received is that of sex cells. In those terms, the female investment greatly exceeds that of male investment as previously mentioned. However, there are other ways in which males invest in their offspring. For example, the male can find food as in the example of balloon flies.[23] He may find a safe environment for the female to feed or lay her eggs as exemplified in many birds.[24][25]
He may also protect the young and provide them with opportunities to learn as in many young as in wolves. Overall, the main role that males overtake is that of protection of the female and their young. That often can decrease the discrepancy of investment caused by the initial investment of sex cells. There are some species such as the Mormon cricket, pipefish seahorse and Panamanian poison arrow frog males invest more. Among the species where the male invests more, the male is also the pickier sex, placing higher demands on their selected female. For example, the female that they often choose usually contain 60% more eggs than rejected females.[26]
This links Parental Investment Theory (PIT) with sexual selection: where parental investment is bigger for a male than a female, it's usually the female who competes for a mate, as shown by Phalaropidae and polyandrous bird species. In these species females are usually more aggressive, brightly colored, and larger than males,[27] suggesting the more investing sex has more choice while selecting a mate compared to the sex engaged in intra-sexual selection.
Females as a valuable resource for males
The second prediction that follows from Trivers' theory is that the fact that women invest more heavily in offspring makes them a valuable resource for males as it ensures the survival of their offspring which is the driving force of natural selection. Therefore, the sex that invests less in offspring will compete among themselves to breed with the more heavily investing sex. In other words, males will compete for females. It has been argued that jealousy has developed to avert the risk of potential loss of parental investment in offspring.[22]
If a male redirects his resources to another female it is a costly loss of time, energy and resources for her offspring. However, the risks for males are higher because although women invest more in their offspring, they have bigger maternity certainty because they themselves have carried out the child. However, males can never have 100% paternal certainty and therefore risk investing resources and time in offspring that is genetically unrelated. Evolutionary psychology views jealousy as an adaptive response to this problem.
Application of Trivers' theory in real life
Trivers' theory has been very influential as the predictions it makes correspond to differences in sexual behaviors of men and women, as demonstrated by a variety of research. Cross-cultural study from Buss (1989)[28] shows that males are tuned into physical attractiveness as it signals youth and fertility and ensures male reproductive success, which is increased by copulating with as many fertile females as possible. Women on the other hand are tuned into resources provided by potential mates, as their reproductive success is increased by ensuring their offspring will survive, and one way they do so is by getting resources for them. Alternatively, another study shows that men are more promiscuous than women, giving further support to this theory. Clark and Hatfield[29] found that 75% of men were willing to have sex with a female stranger when propositioned, compared to 0% of women. On the other hand, 50% of women agreed to a date with a male stranger. This suggests males seek short term relationships, while women show a strong preference for long-term relationships.
However, these preferences (male promiscuity and female choosiness) can be explained in other ways. In Western cultures, male promiscuity is encouraged through the availability of pornographic magazines and videos targeted to the male audience. Alternatively, both Western and Eastern cultures discourage female promiscuity through social checks such as slut-shaming.[30]
PIT (Parental Investment Theory) also explains patterns of sexual jealousy.[22] Males are more likely to show a stress response when imagining their partners showing sexual infidelity (having sexual relations with someone else), and women showed more stress when imagining their partner being emotionally unfaithful (being in love with another woman). PIT explains this, as woman's sexual infidelity decreases the male's paternal certainty, thus he will show more stress due to fear of cuckoldry. On the other hand, the woman fears losing the resources her partner provides. If her partner has an emotional attachment to another female it's likely that he won't invest into their offspring as much, thus a greater stress response is shown in this circumstance.
A heavy criticism of the theory comes from Thornhill and Palmer's analysis of it in A Natural History of Rape: Biological Bases of Sexual Coercion,[31] as it seems to rationalise rape and sexual coercion of females. Thornhill and Palmer claimed rape is an evolved technique for obtaining mates in an environment where women choose mates. As PIT claims males seek to copulate with as many fertile females as possible, the choice women have could result in a negative effect on the male's reproductive success. If women didn't choose their mates, Thornhill and Palmer claim there would be no rape. This ignores a variety of sociocultural factors, such as the fact that not only fertile females are raped – 34% of underage rape victims are under 12,[32] which means they are not of fertile age, thus there is no evolutionary advantage in raping them. 14% of rapes in England are committed on males,[33] who cannot increase a man's reproductive success as there will be no conception. Thus, what Thornhill and Palmer called an 'evolved machinery' might not be very advantageous.
Versus sexual strategies
Trivers' theory overlooks that women do have short-term relationships such as one-night stands, while not all men behave promiscuously. An alternative explanation to PIT (Parental Investment Theory) and mate preferences would be Buss and Schmitt's sexual strategies theory.[34] SST argues that both sexes pursue short-term and long-term relationships, but seek different qualities in their short- and long-term partners. For a short-term relationship women will prefer an attractive partner, but in a long-term relationship they might be willing to trade-off that attractiveness for resources and commitment. On the other hand, men might be accepting of a sexually willing partner in a short-term relationships, but to ensure their paternal certainty they will seek a faithful partner instead.
International politics
Parental investment theory is not only used to explain evolutionary phenomena and human behavior but describes recurrences in international politics as well. Specifically, parental investment is referred to when describing competitive behaviors between states and determining aggressive nature of foreign policies. The parental investment hypothesis states that the size of coalitions and the physical strengths of its male members determines whether its activities with its foreign neighbors are aggressive or amiable.[35] According to Trivers, men have had relatively low parental investments, and were therefore forced into fiercer competitive situations over limited reproductive resources. Sexual selection naturally took place and men have evolved to address its unique reproductive problems. Among other adaptations, men's psychology has also developed to directly aid men in such intra-sexual competition.[35]
One essential psychological developments involved decision-making of whether to take flight or actively engage in warfare with another rivalry group. The two main factors that men referred to in such situations were (1) whether the coalition they are a part of is larger than its opposition and (2) whether the men in their coalition have greater physical strength than the other. The male psychology conveyed in the ancient past has been passed on to modern times causing men to partly think and behave as they have during ancestral wars. According to this theory, leaders of international politics were not an exception. For example, the United States expected to win the Vietnam war due to its greater military capacity when compared to its enemies. Yet victory, according to the traditional rule of greater coalition size, did not come about because the U.S. did not take enough consideration to other factors, such as the perseverance of the local population.[35]
The parental investment hypothesis contends that male physical strength of a coalition still determines the aggressiveness of modern conflicts between states. While this idea may seem unreasonable upon considering that male physical strength is one of the least determining aspects of today's warfare, human psychology has nevertheless evolved to operate on this basis. Moreover, although it may seem that mate seeking motivation is no longer a determinant, in modern wars sexuality, such as rape, is undeniably evident in conflicts even to this day.[35]
.jpg)
Sexual selection
In many species, males can produce a larger number of offspring over the course of their lives by minimizing parental investment in favor of investing time impregnating any reproductive-age female who is fertile. In contrast, a female can have a much smaller number of offspring during her reproductive life, partly due to higher obligate parental investment. Females will be more selective ("choosy") of mates than males will be, choosing males with good fitness (e.g., genes, high status, resources, etc.), so as to help offset any lack of direct parental investment from the male, and therefore increase reproductive success. Robert Trivers' theory of parental investment predicts that the sex making the largest investment in lactation, nurturing, and protecting offspring will be more discriminating in mating; and that the sex that invests less in offspring will compete via intrasexual selection for access to the higher-investing sex (see Bateman's principle[36]).
In species where both sexes invest highly in parental care, mutual choosiness is expected to arise. An example of this is seen in crested auklets, where parents share equal responsibility in incubating their single egg and raising the chick. In crested auklets, both sexes are ornamented.[37]
Parental investment in humans
Humans have evolved increasing levels of parental investment, both biologically and behaviorally. The fetus requires high investment from the mother, and the altricial newborn requires high investment from a community. Species whose newborn young are unable to move on their own and require parental care have a high degree of altriciality. Human children are born unable to care for themselves and require additional parental investment post-birth in order to survive.[38]
Maternal investment
Trivers (1972)[2] hypothesized that greater biologically obligated investment will predict greater voluntary investment. Mothers invest an impressive amount in their children before they are even born. The time and nutrients required to develop the fetus, and the risks associated with both giving these nutrients and undergoing childbirth, are a sizable investment. To ensure that this investment is not for nothing, mothers are likely to invest in their children after they are born, to be sure that they survive and are successful. Relative to most other species, human mothers give more resources to their offspring at a higher risk to their own health, even before the child is born. This is associated with the evolution of a slower life history, in which fewer, larger offspring are born after longer intervals, requiring increased parental investment.[39][40]

The developing human fetus––and especially the brain––requires nutrients to grow. In the later weeks of gestation, the fetus requires increasing nutrients as the growth of the brain increases.[41] Rodents and primates have the most invasive placenta phenotype, the hemochorial placenta, in which the chorion erodes the uterine epithelium and has direct contact with maternal blood. The other placental phenotypes are separated from the maternal bloodstream by at least one layer of tissue. The more invasive placenta allows for a more efficient transfer of nutrients between the mother and fetus, but it comes with risks as well. The fetus is able to release hormones directly into the mother’s bloodstream to “demand” increased resources. This can result in health problems for the mother, such as pre-eclampsia. During childbirth, the detachment of the placental chorion can cause excessive bleeding.[42]
The obstetrical dilemma also makes birth more difficult and results in increased maternal investment. Humans have evolved both bipedalism and large brain size. The evolution of bipedalism altered the shape of the pelvis, and shrunk the birth canal at the same time brains were evolving to be larger. The decreasing birth canal size meant that babies are born earlier in development, when they have smaller brains. Humans give birth to babies with brains 25% developed, while other primates give birth to offspring with brains 45-50% developed.[43] A second possible explanation for the early birth in humans is the energy required to grow and sustain a larger brain. Supporting a larger brain gestationally requires energy the mother may be unable to invest.[44]
The obstetrical dilemma makes birth challenging, and a distinguishing trait of humans is the need for assistance during childbirth. The altered shape of the bipedal pelvis requires that babies leave the birth canal facing away from the mother, contrary to all other primate species. This makes it more difficult for the mother to clear the baby’s breathing passageways, to make sure the umbilical cord isn’t wrapped around the neck, and to pull the baby free without bending its body the wrong way.[45]
The human need to have a birth attendant also requires sociality. In order to guarantee the presence of a birth attendant, humans must aggregate in groups. It has been controversially claimed that humans have eusociality,[46] like ants and bees, in which there is relatively high parental investment, cooperative care of young, and division of labor. It is unclear which evolved first; sociality, bipedalism, or birth attendance. Bonobos, our closest living relatives alongside chimpanzees, have high female sociality and births among bonobos are also social events.[47][48] Sociality may have been a prerequisite for birth attendance, and bipedalism and birth attendance could have evolved as long as five million years ago.[38]
As female primates age, their ability to reproduce decreases. The grandmother hypothesis describes the evolution of menopause, which may or may not be unique to humans among primates.[49] As women age, the costs of investing in additional reproduction increase and the benefits decrease. At menopause, it is more beneficial to stop reproduction and begin investing in grandchildren. Grandmothers are certain of their genetic relation to their grandchildren, especially the children of their daughters, because maternal certainty of their own children is high, and their daughters are certain of their maternity to their children as well. It has also been theorized that grandmothers preferentially invest in the daughters of their daughters because X chromosomes carry more DNA and their granddaughters are most closely related to them.[50]
Paternal investment
As altriciality increased, investment from individuals other than the mother became more necessary. High sociality meant that female relatives were present to help the mother, but paternal investment increased as well. Paternal investment increases as it becomes more difficult to have additional children, and as the effects of investment on offspring fitness increase.[51]
Men are more likely than women to give no parental investment to their children, and the children of low-investing fathers are more likely to give less parental investment to their own children. Father absence is a risk factor for both early sexual activity and teenage pregnancy.[52][53][54][55] Father absence raises children's stress levels, which are linked to earlier onset of sexual activity and increased short-term mating orientation.[56][57][58][59][60] Daughters of absent fathers are more likely to seek short-term partners, and one theory explains this as a preference for outside (non-partner) social support because of the perceived uncertain future and uncertain availability of committing partners in a high-stress environment.[61]
Investment as predictor of mating strategies
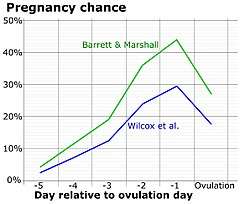
Concealed ovulation
Women can only get pregnant while ovulating. Human ovulation is concealed, or not signaled externally. Concealed ovulation decreases paternity certainty because men are unsure when women ovulate.[62] The evolution of concealed ovulation has been theorized to be a result of altriciality and increased need for paternal investment. There are two ways this could be true. First, if men are unsure of the time of ovulation, the best way to successfully reproduce would be to repeatedly mate with a woman throughout her cycle, which requires pair bonding, which in turn increases paternal investment.[63] The second theory states that decreased paternity certainty would increase paternal investment in polygamous groups, because more men may invest in the offspring. The second theory is better regarded today, because all mammals with concealed ovulation are promiscuous, and men display relatively low mate-guarding behavior, as monogamy and the first theory require.[64]
Mating orientations
Sociosexuality was first described by Alfred Kinsey as a willingness to engage in casual and uncommitted sexual relationships.[65] Sociosexual orientation describes sociosexuality on a scale from unrestricted to restricted. Individuals with an unrestricted sociosexual orientation have higher openness to sex in less committed relationships, and individuals with a restricted sociosexual orientation have lower openness to casual sexual relationships.[66][67] However, today it is acknowledged that sociosexuality does not in reality exist on a one-dimensional scale. Individuals who are less open to casual relationships are not always seeking committed relationships, and individuals who are less interested in committed relationships are not always interested in casual relationships.[68] Short- and long-term mating orientations are the modern descriptors of openness to uncommitted and committed relationships, respectively.[69]
Parental investment theory, as proposed by Trivers, argues that the sex with higher obligatory investment will be more selective in choosing sex partners, and the sex with lower obligatory investment will be less selective and more interested in "casual" mating opportunities. The more investing sex cannot reproduce as frequently, causing the less investing sex to compete for mating opportunities.[21][70] In humans, women have higher obligatory investment (pregnancy and childbirth), than men (sperm production).[34] Women are more likely to have higher long-term mating orientations, and men are more likely to have higher short-term mating orientations.[68]
Short- and long-term mating orientations influence women's preferences in men. Studies have found that women put great emphasis on career-orientation, ambition and devotion only when considering a long-term partner.[71] When marriage is not involved, women put greater emphasis on physical attractiveness.[72] Generally, women prefer men who are likely to perform high parental investment and have good genes. Women prefer men with good financial status, who are more committed, who are more athletic, and who are healthier.[73]
Some inaccurate theories have been inspired by parental investment theory. The "structural powerlessness hypothesis"[74] proposes that women strive to find mates with access to high levels of resources because as women, they are excluded from these resources directly. However, this hypothesis has been disproved by studies which found that financially successful women place an even greater importance on financial status, social status, and possession of professional degrees.[75]

Sexual dimorphism
Sexual dimorphism is the difference in body size between male and female members of a species as a result of intrasexual selection, which is sexual selection that acts within a sex. High sexual dimorphism and larger body size in males is a result of male-male competition for females.[76] Primate species in which groups are formed of many females and one male have higher sexual dimorphism than species that have both multiple females and males, or one female and one male. Polygynous primates have the highest sexual dimorphism, and polygamous and monogamous primates have less.[77][78] Humans have the lowest levels of sexual dimorphism of any primate species, indicating that we have evolved decreasing levels of polygyny. Decreased polygyny is associated with increased paternal investment.[79][80]
The demographic transition
The demographic transition describes the modern decrease in both birth and death rates. From a Darwinian perspective, it does not make sense that families with more resources are having fewer children. One explanation for the demographic transition is the increased parental investment required to raise children who will be able to maintain the same level of resources as their parents.[81]
See also
| Wikimedia Commons has media related to Parental care in animals. |
References
- Clutton-Brock, T.H. 1991. The Evolution of Parental Care. Princeton, NJ: Princeton U. Press. pg. 9
- Trivers, R.L. (1972). Parental investment and sexual selection. In B. Campbell (Ed.), Sexual selection and the descent of man, 1871-1971 (pp. 136–179). Chicago, IL: Aldine. ISBN 0-435-62157-2.
- Darwin, Charles (2009), "The Publication of the 'origin of Species'—Oct. 3, 1859–Dec. 31, 1859", in Darwin, Francis (ed.), The Life and Letters of Charles Darwin, Cambridge University Press, pp. 205–255, doi:10.1017/cbo9780511702891.007, ISBN 9780511702891
- Fisher, Ronald Aylmer (1930). The genetical theory of natural selection. Oxford: Clarendon Press. doi:10.5962/bhl.title.27468.
- Bateman, A J (December 1948). "Intra-sexual selection in Drosophila". Heredity. 2 (3): 349–368. doi:10.1038/hdy.1948.21. ISSN 0018-067X. PMID 18103134.
- TRIVERS, ROBERT L. (February 1974). "Parent-Offspring Conflict". American Zoologist. 14 (1): 249–264. doi:10.1093/icb/14.1.249. ISSN 0003-1569.
- Edwards, A. W. F. (1 April 2000). "The Genetical Theory of Natural Selection". Genetics. 154 (4): 1419–1426. PMC 1461012. PMID 10747041 – via www.genetics.org.
- Burke, T.; Daviest, N. B.; Bruford, M. W.; Hatchwell, B. J. (1989). "Parental care and mating behaviour of polyandrous dunnocks Prunella modularis related to paternity by DNA fingerprinting". Nature. 338 (6212): 249–51. Bibcode:1989Natur.338..249B. doi:10.1038/338249a0.
- Santos, R. S. (1995). "Allopaternal care in redlip blenny". Journal of Fish Biology. 47 (2): 350–353. doi:10.1111/j.1095-8649.1995.tb01904.x.
- Kelly, Caitlin A.; Norbutus, Amanda J.; Lagalante, Anthony F.; Iyengar, Vikram K. (2012). "Male courtship pheromones as indicators of genetic quality in an arctiid moth (Utetheisa ornatrix)". Behavioral Ecology. 23 (5): 1009–14. doi:10.1093/beheco/ars064.
- Schürch, Roger, Roger & Taborsky, Barbara (2005). "The Functional Significance of Buccal Feeding in the Mouthbrooding Cichlid Tropheus moorii". Behaviour. 142 (3): 265–281. doi:10.1163/1568539053778274. JSTOR 4536244.
- Davies, Nicholas B.; John R. Krebs & Stuart A. West (2012). An Introduction to Behavioral Ecology. Wiley-Blackwell. pp. 367–371.
- Barett, L., Dunbar, R. & Lycett, J. (2002). Human Evolutionary Psychology. Palgrave Press.
- Olsson, Olof (1997). "Clutch abandonment: a state-dependent decision in king penguins". Journal of Avian Biology. 28 (3): 264–267. doi:10.2307/3676979. JSTOR 3676979.
- Salmon, Catherine A.; Shackelford, Todd K. (September 2007). Family Relationships: An Evolutionary Perspective. Oxford Scholarship Online. ISBN 9780195320510.
- Scheper-Hughes, Nancy (December 1985). "Culture, Scarcity, and Maternal Thinking: Maternal Detachment and Infant Survival in a Brazilian Shantytown". Ethos. 13 (4): 291–317. doi:10.1525/eth.1985.13.4.02a00010. ISSN 0091-2131.
- Solomon, Nancy G., and Loren D. Hayes. “The Biological Basis of Alloparental Behaviour in Mammals.” Substitute Parents: Biological and Social Perspectives on Alloparenting in Human Societies, edited by Gillian Bentley and Ruth Mace, NED - New edition, 1 ed., Berghahn Books, 2009, pp. 13–49. JSTOR, www.jstor.org/stable/j.ctt9qch9m.7.
- Hrdy, Sarah Blaffer. “WHY IT TAKES A VILLAGE.” Mothers and Others, Harvard University Press, Cambridge, Massachusetts; London, England, 2009, pp. 65–110. JSTOR, www.jstor.org/stable/j.ctt1c84czb.5.
- Morgan, C. L. (1894). An introduction to comparative psychology. London: W. Scott.
- Epstein, R. (1984). The principle of parsimony and some applications in psychology. Journal of Mind and Behavior, 5
- Trivers, R. (1972). Parental investment and sexual selection. Sexual Selection & the Descent of Man, Aldine de Gruyter, New York, 136-179.
- Buss, D. M.; Larsen, R. J.; Westen, D.; Semmelroth, J. (1992). "Sex differences in jealousy: Evolution, physiology, and psychology" (PDF). Psychological Science. 3 (4): 251–255. doi:10.1111/j.1467-9280.1992.tb00038.x.
- Kessel, E. L. (1955). "The mating activities of balloon flies". Systematic Zoology. 4 (3): 97–104. doi:10.2307/2411862. JSTOR 2411862.
- Royama, T (1966). "A re-interpretation of courtship feeding". Bird Study. 13 (2): 116–129. doi:10.1080/00063656609476115.
- Stokes, A. W., & Williams, H. W. (1971). Courtship feeding in gallinaceous birds. The Auk, 543-559.Chicago
- Trivers, R. (1985). Social evolution. Menlo Park, CA: Benjamin/Cummings.
- Lack, D. L. (1968). Ecological adaptations for breeding in birds.
- Buss, D. M. (1989). "Sex differences in human mate preferences: Evolutionary hypotheses tested in 37 cultures" (PDF). Behavioral and Brain Sciences. 12 (1): 1–14. doi:10.1017/s0140525x00023992.
- Clark, R. D.; Hatfield, E. (1989). "Gender differences in receptivity to sexual offers" (PDF). Journal of Psychology & Human Sexuality. 2 (1): 39–55. doi:10.1300/j056v02n01_04.
- Armstrong, E. A.; Hamilton, L. T.; Armstrong, E. M.; Seeley, J. L. (2014). ""Good Girls" Gender, Social Class, and Slut Discourse on Campus" (PDF). Social Psychology Quarterly. 77 (2): 100–122. doi:10.1177/0190272514521220.
- Thornhill, R., & Palmer, C. T. (2001). A natural history of rape: Biological bases of sexual coercion. MIT press.
- "Children and Teens: Statistics - RAINN".
- "Rape statistics".
- Buss, D. M.; Schmitt, D. P. (1993). "Sexual strategies theory: an evolutionary perspective on human mating" (PDF). Psychological Review. 100 (2): 204–32. doi:10.1037/0033-295x.100.2.204. PMID 8483982.
- Lopez, Anthony C.; McDermott, Rose; Petersen, Michael Bang (2011). "States in Mind: Evolution, Coalitional Psychology, and International Politics". International Security. 36 (2): 48–83. doi:10.1162/isec_a_00056.
- Bateman AJ (December 1948). "Intra-sexual selection in Drosophila". Heredity. 2 (3): 349–68. doi:10.1038/hdy.1948.21. PMID 18103134.
- Amundsen, Trond (1 April 2000). "Why are female birds ornamented". Trends in Ecology & Evolution. 15 (4): 149–55. doi:10.1016/S0169-5347(99)01800-5. PMID 10717684.
- Rosenberg, Karen; Trevathan, Wenda (November 2002). "Birth, obstetrics and human evolution". BJOG: An International Journal of Obstetrics and Gynaecology. 109 (11): 1199–1206. doi:10.1046/j.1471-0528.2002.00010.x. ISSN 1470-0328. PMID 12452455.
- Garratt, M.; Gaillard, J.-M.; Brooks, R. C.; Lemaitre, J.-F. (2013-04-22). "Diversification of the eutherian placenta is associated with changes in the pace of life". Proceedings of the National Academy of Sciences. 110 (19): 7760–7765. Bibcode:2013PNAS..110.7760G. doi:10.1073/pnas.1305018110. ISSN 0027-8424. PMC 3651450. PMID 23610401.
- Bielby, J.; Mace, G. M.; Bininda‐Emonds, O. R. P.; Cardillo, M.; Gittleman, J. L.; Jones, K. E.; Orme, C. D. L.; Purvis, A. (June 2007). "The Fast‐Slow Continuum in Mammalian Life History: An Empirical Reevaluation". The American Naturalist. 169 (6): 748–757. doi:10.1086/516847. ISSN 0003-0147. PMID 17479461.
- Soares, Michael J; Varberg, Kaela M; Iqbal, Khursheed (2018-02-22). "Hemochorial placentation: development, function, and adaptations†". Biology of Reproduction. 99 (1): 196–211. doi:10.1093/biolre/ioy049. ISSN 0006-3363. PMC 6044390. PMID 29481584.
- Mahmoud F. Fathalla (2013). "How Evolution of the Human Brain Shaped Women's Sexual and Reproductive Health". Reproductive Biology Insights. 6: 11–18. doi:10.4137/rbi.s12217. ISSN 1178-6426.
- Wenda., Trevathan (2011). Human birth : an evolutionary perspective. New Brunswick: Transaction Publishers. ISBN 9781412815024. OCLC 669122326.
- Wells, Jonathan C.K.; DeSilva, Jeremy M.; Stock, Jay T. (2012). "The obstetric dilemma: An ancient game of Russian roulette, or a variable dilemma sensitive to ecology?". American Journal of Physical Anthropology. 149 (S55): 40–71. doi:10.1002/ajpa.22160. ISSN 0002-9483. PMID 23138755.
- Trevathan, Wenda R. (June 1996). "The Evolution of Bipedalism and Assisted Birth". Medical Anthropology Quarterly. 10 (2): 287–290. doi:10.1525/maq.1996.10.2.02a00100. ISSN 0745-5194. PMID 8744088.
- Gintis, Herbert (November 2012). "Clash of the Titans". BioScience. 62 (11): 987–991. doi:10.1525/bio.2012.62.11.8. ISSN 1525-3244.
- Demuru, Elisa; Ferrari, Pier Francesco; Palagi, Elisabetta (September 2018). "Is birth attendance a uniquely human feature? New evidence suggests that Bonobo females protect and support the parturient". Evolution and Human Behavior. 39 (5): 502–510. doi:10.1016/j.evolhumbehav.2018.05.003. ISSN 1090-5138.
- Douglas, Pamela Heidi (2014-07-10). "Female sociality during the daytime birth of a wild bonobo at Luikotale, Democratic Republic of the Congo". Primates. 55 (4): 533–542. doi:10.1007/s10329-014-0436-0. ISSN 0032-8332. PMID 25007717.
- Walker, Margaret L.; Herndon, James G. (2008-09-01). "Menopause in Nonhuman Primates?1". Biology of Reproduction. 79 (3): 398–406. doi:10.1095/biolreprod.108.068536. ISSN 0006-3363. PMC 2553520. PMID 18495681.
- Fox, Molly; Johow, Johannes; Knapp, Leslie A. (2011). "The Selfish Grandma Gene: The Roles of the X-Chromosome and Paternity Uncertainty in the Evolution of Grandmothering Behavior and Longevity". International Journal of Evolutionary Biology. 2011: 165919. doi:10.4061/2011/165919. ISSN 2090-052X. PMC 3118636. PMID 21716697.
- Tattersall, Ian (2010-08-10). "Paleoanthropology: Two New Offerings". Evolution: Education and Outreach. 3 (3): 464–465. doi:10.1007/s12052-010-0263-8. ISSN 1936-6426.
- McLanahan, S. S. (1999). Father absence and the welfare of children. Coping with divorce, single parenting, and remarriage: A risk and resiliency perspective, 117-145.
- Kiernan, K. E.; Hobcraft, J. (1997). "Parental divorce during childhood: age at first intercourse, partnership and parenthood" (PDF). Population Studies. 51 (1): 41–55. doi:10.1080/0032472031000149716.
- Ellis, B. J.; Bates, J. E.; Dodge, K. A.; Fergusson, D. M.; John Horwood, L.; Pettit, G. S.; Woodward, L. (2003). "Does father absence place daughters at special risk for early sexual activity and teenage pregnancy?". Child Development. 74 (3): 801–821. doi:10.1111/1467-8624.00569. PMC 2764264. PMID 12795391.
- Scaramella, L. V.; Conger, R. D.; Simons, R. L.; Whitbeck, L. B. (1998). "Predicting risk for pregnancy by late adolescence: a social contextual perspective" (PDF). Developmental Psychology. 34 (6): 1233. doi:10.1037/0012-1649.34.6.1233.
- Belsky, J.; Steinberg, L.; Draper, P. (1991). "Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization". Child Development. 62 (4): 647–670. doi:10.2307/1131166. JSTOR 1131166.
- Coley, R. L.; Chase-Lansdale, P. L. (1998). "Adolescent pregnancy and parenthood: recent evidence and future directions" (PDF). American Psychologist. 53 (2): 152–66. doi:10.1037/0003-066x.53.2.152. PMID 9491745.
- Antfolk, J.; Sjölund, A. (2018). "High parental investment in childhood is associated with increased mate value in adulthood" (PDF). Personality and Individual Differences. 127 (1): 144–150. doi:10.1016/j.paid.2018.02.004.
- Lynn, D. B.; Sawrey, W. L. (1959). "The effects of father-absence on Norwegian boys and girls". The Journal of Abnormal and Social Psychology. 59 (2): 258–62. doi:10.1037/h0040784. PMID 14419160.
- Malamuth, N. M. (1981). "Rape proclivity among males" (PDF). Journal of Social Issues. 37 (4): 138–157. doi:10.1111/j.1540-4560.1981.tb01075.x.
- Chisholm, J. S.; Quinlivan, J. A.; Petersen, R. W.; Coall, D. A. (2005). "Early stress predicts age at menarche and first birth, adult attachment, and expected lifespan" (PDF). Human Nature. 16 (3): 233–265. doi:10.1007/s12110-005-1009-0. PMID 26189749.
- Sillen-Tullberg, Birgitta; Moller, Anders P. (January 1993). "The Relationship between Concealed Ovulation and Mating Systems in Anthropoid Primates: A Phylogenetic Analysis". The American Naturalist. 141 (1): 1–25. doi:10.1086/285458. ISSN 0003-0147. PMID 19426020.
- BENSHOOF, L; THORNHILL, R (April 1979). "The evolution of monogamy and concealed ovulation in humans". Journal of Social and Biological Systems. 2 (2): 95–106. doi:10.1016/0140-1750(79)90001-0. ISSN 0140-1750.
- 1962-, Ryan, Christopher (2012). Sex at dawn : how we mate, why we stray, and what it means for modern relationships. Jethá, Cacilda. New York: Harper. ISBN 9780062207944. OCLC 793342018.CS1 maint: numeric names: authors list (link)
- Kinsey, Alfred C.; Pomeroy, Wardell R.; Martin, Clyde E. (June 2003). "Sexual Behavior in the Human Male". American Journal of Public Health. 93 (6): 894–898. doi:10.2105/ajph.93.6.894. ISSN 0090-0036. PMC 1643237. PMID 12773346.
- Gangestad, S. W.; Simpson, J. A.; DiGeronimo, K.; Biek, M. (1992). "Differential accuracy in person perception across traits: examination of a functional hypothesis" (PDF). Journal of Personality and Social Psychology. 62 (4): 688. doi:10.1037/0022-3514.62.4.688.
- Simpson, J. A.; Gangestad, S. W. (1991). "Individual differences in sociosexuality: evidence for convergent and discriminant validity". Journal of Personality and Social Psychology. 60 (6): 870. doi:10.1037/0022-3514.60.6.870.
- Holtzman, Nicholas S.; Strube, Michael J. (2013-07-18). "Above and beyond Short-Term Mating, Long-Term Mating is Uniquely Tied to Human Personality". Evolutionary Psychology. 11 (5): 147470491301100. doi:10.1177/147470491301100514. ISSN 1474-7049.
- Jackson, Jenée James; Kirkpatrick, Lee A. (November 2007). "The structure and measurement of human mating strategies: toward a multidimensional model of sociosexuality". Evolution and Human Behavior. 28 (6): 382–391. doi:10.1016/j.evolhumbehav.2007.04.005. ISSN 1090-5138.
- Wade, M. J.; Shuster, S. M. (2002). "The evolution of parental care in the context of sexual selection: a critical reassessment of parental investment theory". The American Naturalist. 160 (3): 285–292. doi:10.1086/341520. PMID 18707439.
- Buss, D. M.; Schmitt, D. P. (1993). "Sexual strategies theory: An evolutionary perspective on human mating" (PDF). Psychological Review. 100 (2): 204–232. doi:10.1037/0033-295x.100.2.204. PMID 8483982.
- Scheib, J. E. (1997, June). Context-specific mate choice criteria: Women’s trade-offs in the contexts of long-term and extra-pair mateships. Paper presented to the Annual Meeting of the Human Behavior and Evolution Society, University of Arizona, Tucson, AZ.
- Buss, M. D. (1999). Evolutionary Psychology: The New Science of the mind. (2nd ed.). United States: Pearson Education, Inc
- David, M. B.; Barnes, M. (1986). "Preferences in Human Mate Selection" (PDF). Journal of Personality and Social Psychology. 50: 559–570. doi:10.1037/0022-3514.50.3.559.
- Buss, D. M. (1989). "Sex differences in human mate preferences: Evolutionary hypotheses testing in 37 cultures" (PDF). Behavioral and Brain Sciences. 12: 1–49. doi:10.1017/s0140525x00023992.
- Lande, Russell (March 1980). "Sexual Dimorphism, Sexual Selection, and Adaptation in Polygenic Characters". Evolution. 34 (2): 292–305. doi:10.2307/2407393. ISSN 0014-3820. JSTOR 2407393. PMID 28563426.
- Cheverud, James M.; Dow, Malcolm M.; Leutenegger, Walter (November 1985). "The Quantitative Assessment of Phylogenetic Constraints in Comparative Analyses: Sexual Dimorphism in Body Weight Among Primates". Evolution. 39 (6): 1335–1351. doi:10.1111/j.1558-5646.1985.tb05699.x. ISSN 0014-3820. PMID 28564267.
- Leutenegger, Walter; Kelly, James T. (January 1977). "Relationship of sexual dimorphism in canine size and body size to social, behavioral, and ecological correlates in anthropoid primates". Primates. 18 (1): 117–136. doi:10.1007/bf02382954. ISSN 0032-8332.
- Leutenegger, Walter; Cheverud, James (December 1982). "Correlates of sexual dimorphism in primates: Ecological and size variables". International Journal of Primatology. 3 (4): 387–402. CiteSeerX 10.1.1.536.6939. doi:10.1007/bf02693740. ISSN 0164-0291.
- Ralls, Katherine (September 1977). "Sexual Dimorphism in Mammals: Avian Models and Unanswered Questions". The American Naturalist. 111 (981): 917–938. doi:10.1086/283223. ISSN 0003-0147.
- Borgerhoff Mulder, Monique (July 1998). "The demographic transition: are we any closer to an evolutionary explanation?". Trends in Ecology & Evolution. 13 (7): 266–270. doi:10.1016/s0169-5347(98)01357-3. ISSN 0169-5347. PMID 21238295.
Further reading
- Clutton-Brock, T.H. and C. Godfray. 1991. "Parental investment," in Behavioural Ecology: An Evolutionary Approach. Edited by J.R. Krebs and N.B. Davies, pp. 234–262. Boston: Blackwell.
- Buss, David M. (March 1989). "Sex differences in human mate preferences: Evolutionary hypotheses tested in 37 cultures". Behavioral and Brain Sciences. 12 (1): 1–14. doi:10.1017/S0140525X00023992.
- Belsky J, Steinberg L, Draper P (August 1991). "Childhood experience, interpersonal development, and reproductive strategy: and evolutionary theory of socialization". Child Development. 62 (4): 647–70. doi:10.1111/j.1467-8624.1991.tb01558.x. PMID 1935336.
- Woodward, Kevin; Richards, Miriam H. (2005). "The parental investment model and minimum mate choice criteria in humans". Behavioral Ecology. 16 (1): 57–61. doi:10.1093/beheco/arh121.
- Geary, D. C. (2005). Evolution of paternal investment. In D. M. Buss (Ed.), The handbook of evolutionary psychology (pp. 483–505). Hoboken, NJ: John Wiley & Sons. Full text