Methylthioirontricarbonyl dimer
Methylthioirontricarbonyl dimer, also known as methanethiolatoirontricarbonyl dimer, is an organometallic compound with the formula Fe2(SCH3)2(CO)6. It is a red volatile solid that is classified as a transition metal thiolate complex. It exists as air-stable red crystals with two isomers, where the methyl groups are either anti (isomer A) or syn (isomer B) with respect to each other.[1]
 | |
 | |
2(CO)6.jpg) | |
| Names | |
|---|---|
| Other names
Methanethiolatoirontricarbonyl dimer | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.035.396 |
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| C8H6Fe2O6S2 | |
| Molar mass | 373.94 g/mol |
| Appearance | red crystals |
| Melting point | 65 °C (149 °F; 338 K) (isomer A), 102 °C (isomer B) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
It was first synthesized 1940[2] with the discovery of isomers in 1962.[3] Synthesis involves treating triiron dodecacarbonyl with dimethyl disulfide:
- 2 Fe3(CO)12 + 3 (CH3)2S2 → 3 [Fe(CO)3SCH3]2 + 6 CO
It can be purified by recrystallization or by sublimation. The isomers can be separated by chromatography.
Structure
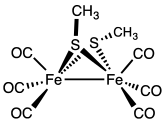
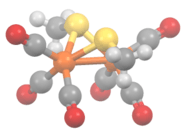
The methylthioirontricarbonyl dimer is a butterfly cluster compound, consisting of two iron atoms with distorted square pyramidal coordination geometry. The geometry is octahedral if the Fe-Fe bond is included. Each iron has three terminal carbon monoxide ligands and two bridging methylthiolate ligands. The Fe-Fe distance is 2.537 Å with an average Fe-S bond length of 2.259 Å. The average Fe-S-Fe bond angle is relatively small at 68.33°. Three isomers are possible but only the diequatorial and axial-equatorial isomers are seen. The diaxial isomer is disfavored due to steric hindrance.[4][5] The structurally related compound [Fe(CO)3S]2 has idealized C2v symmetry.
References
- King, R. B. "Organometallic Synthesis, Volume 1: Transition-metal compounds" (1965) Academic Press. ISBN 0124080502
- Hieber, W., Scharfenberg, C. "Einwirkung organischer Schwefelverbindungn auf die Carbonyle des Eisens" Chem. Ber. 1949, vol. 73, 1012. doi:10.1002/cber.19400730914
- R. B. King (1962). "Organosulfur Derivatives of Metal Carbonyls. I. The Isolation of Two Isomeric Products in the Reaction of Triiron Dodecacarbonyl with Dimethyl Disulfide". J. Am. Chem. Soc. 84: 2460. doi:10.1021/ja00871a045.
- Dahl, L. F., Wei, C. H. (1963). "Structure and Nature of Bonding of [C2H5SFe(CO)3]2". Inorg. Chem. 2: 328. doi:10.1021/ic50006a022.CS1 maint: uses authors parameter (link)
- M. C. Ortega-Alfaro, N. Hernández, I. Cerna, J. G. López-Cortés, E. Gómez, R. A. Toscano, C. Alvarez-Toledano (2004). "Novel dinuclear iron(0) complexes from α,β-unsaturated ketones β-positioned with sulfide and sulfoxide groups". J. Organomet. Chem. 689: 885–893. doi:10.1016/j.jorganchem.2003.12.015.CS1 maint: uses authors parameter (link)