Methyl azide
Methyl azide is a covalent molecule related to hydrazoic acid and other alkyl azides.
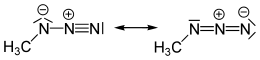 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Azidomethane | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| CH3N3 | |||
| Molar mass | 57.05 | ||
| Appearance | white powder | ||
| slightly soluble | |||
| Solubility | alkane, ether | ||
| Explosive data | |||
| Shock sensitivity | High | ||
| Friction sensitivity | High | ||
| Hazards | |||
| Main hazards | Highly explosive | ||
| Related compounds | |||
Related compounds |
Hydrazoic acid, Chlorine azide, Ethyl azide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation and properties
Methyl azide can be prepared by the methylation of sodium azide, for instance with dimethyl sulfate in alkaline solution, followed by passing through a tube of anhydrous calcium chloride or sodium hydroxide to remove contaminating hydrazoic acid.[1] The first synthesis was reported in 1905.[2]
It decomposes in a first-order reaction:[3]
- CH3N3 → CH3N + N2
Methyl azide might be a potential precursor in the synthesis of prebiotic molecules via nonequilibrium reactions on interstellar ices initiated by energetic galactic cosmic rays (GCR) and photons.[4]
Safety precautions
Methyl azide is stable at ambient temperature but may explode when heated. Presence of mercury increases the sensitivity to shock and spark. It is incompatible with methanol and dimethyl malonate.[5] When heated to decomposition, it emits toxic fumes of NOx. It can be stored indefinitely in the dark at −80 °C.[1]
References
- Chae, Junghyun (2008-03-14), John Wiley & Sons, Ltd (ed.), "Methyl Azide", Encyclopedia of Reagents for Organic Synthesis, Chichester, UK: John Wiley & Sons, Ltd, pp. rn00795, doi:10.1002/047084289x.rn00795, ISBN 978-0-471-93623-7, retrieved 2020-08-10
- Dimroth, O.; Wislicenus, W. (1905). "Ueber das Methylazid" (PDF). Berichte der Deutschen Chemischen Gesellschaft. 38 (2): 1573–1576. doi:10.1002/cber.19050380254.
- O'Dell, M. S.; Darwent, B. (1970). "Thermal decomposition of methyl azide". Canadian Journal of Chemistry. 48 (7): 1140–1147. doi:10.1139/v70-187.
- Quinto-Hernandez, A.; Wodtke, A. M.; Bennett, C. J.; Kim, Y. S.; Kaiser, R. I. (2011). "On the Interaction of Methyl Azide (CH3N3) Ices with Ionizing Radiation: Formation of Methanimine (CH2NH), Hydrogen Cyanide (HCN), and Hydrogen Isocyanide (HNC)". The Journal of Physical Chemistry A. 115 (3): 250–264. doi:10.1021/jp103028v. PMID 21162584.
- Urben, P. G., ed. (2006). Bretherick's Handbook of Reactive Chemical Hazards (7th ed.). Elsevier. ISBN 9780123725639.
External links
- Graner, G.; Hirota, E.; Iijima, T.; Kuchitsu, K.; Ramsay, D. A.; Vogt, J.; Vogt, N. "CH3N3 Methyl azide". In Kuchitsu, K. (ed.). Group II Molecules and Radicals: Numerical Data and Functional Relationships in Science and Technology. 25 B. doi:10.1007/10653318_320.
- "Methyl azide". NIST Webbook. National Institute for Standards and Technology.
Salts and covalent derivatives of the azide ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HN3 | He | ||||||||||||||||||
| LiN3 | Be(N3)2 | B(N3)3 | CH3N3, C(N3)4 |
N(N3)3,H2N—N3 | O | FN3 | Ne | ||||||||||||
| NaN3 | Mg(N3)2 | Al(N3)3 | Si(N3)4 | P | SO2(N3)2 | ClN3 | Ar | ||||||||||||
| KN3 | Ca(N3)2 | Sc(N3)3 | Ti(N3)4 | VO(N3)3 | Cr(N3)3, CrO2(N3)2 |
Mn(N3)2 | Fe(N3)2, Fe(N3)3 |
Co(N3)2, Co(N3)3 |
Ni(N3)2 | CuN3, Cu(N3)2 |
Zn(N3)2 | Ga(N3)3 | Ge | As | Se(N3)4 | BrN3 | Kr | ||
| RbN3 | Sr(N3)2 | Y | Zr(N3)4 | Nb | Mo | Tc | Ru(N3)63− | Rh(N3)63− | Pd(N3)2 | AgN3 | Cd(N3)2 | In | Sn | Sb | Te | IN3 | Xe(N3)2 | ||
| CsN3 | Ba(N3)2 | Hf | Ta | W | Re | Os | Ir(N3)63− | Pt(N3)62− | Au(N3)4− | Hg2(N3)2, Hg(N3)2 |
TlN3 | Pb(N3)2 | Bi(N3)3 |
Po | At | Rn | |||
| Fr | Ra(N3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La | Ce(N3)3, Ce(N3)4 |
Pr | Nd | Pm | Sm | Eu | Gd(N3)3 | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||
| Ac | Th | Pa | UO2(N3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||