MMP10
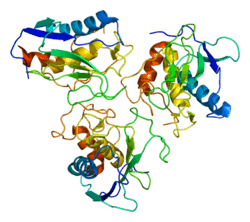
Stromelysin-2 also known as matrix metalloproteinase-10 (MMP-10) or transin-2 is an enzyme that in humans is encoded by the MMP10 gene.[5][6]
Function
Proteins of the matrix metalloproteinase (MMP) family are involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, and tissue remodeling, as well as in disease processes, such as arthritis and metastasis. Most MMP's are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. The enzyme encoded by this gene degrades proteoglycans and fibronectin. The gene is part of a cluster of MMP genes which localize to chromosome 11q22.3.[7]
Clinical significance
MMP10 has been linked to cancer stem cell vitality and metastasis.[8]
MMP10 is a potential prognostic biomarker for oral cancer.[9]
References
- GRCh38: Ensembl release 89: ENSG00000166670 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000047562 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Muller D, Quantin B, Gesnel MC, Millon-Collard R, Abecassis J, Breathnach R (July 1988). "The collagenase gene family in humans consists of at least four members". The Biochemical Journal. 253 (1): 187–92. doi:10.1042/bj2530187. PMC 1149273. PMID 2844164.
- Jung JY, Warter S, Rumpler Y (1990). "Localization of stromelysin 2 gene to the q22.3-23 region of chromosome 11 by in situ hybridization". Annales de Génétique. 33 (1): 21–3. PMID 2369069.
- "Entrez Gene: MMP10 matrix metallopeptidase 10 (stromelysin 2)".
- Justilien V, Regala RP, Tseng IC, Walsh MP, Batra J, Radisky ES, Murray NR, Fields AP (2012). "Matrix metalloproteinase-10 is required for lung cancer stem cell maintenance, tumor initiation and metastatic potential". PLOS ONE. 7 (4): e35040. doi:10.1371/journal.pone.0035040. PMC 3335833. PMID 22545096.
- Upadhyay P, Gardi N, Desai S, Chandrani P, Joshi A, Dharavath B, Arora P, Bal M, Nair S, Dutt A (2017). "Genomic characterization of tobacco/nut chewing HPV-negative early stage tongue tumors identify MMP10 asa candidate to predict metastases". Oral Oncology. 73: 56–64. doi:10.1016/j.oraloncology.2017.08.003. PMC 5628952. PMID 28939077.
Further reading
- Chandler S, Miller KM, Clements JM, Lury J, Corkill D, Anthony DC, Adams SE, Gearing AJ (February 1997). "Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: an overview". Journal of Neuroimmunology. 72 (2): 155–61. doi:10.1016/S0165-5728(96)00179-8. PMID 9042108.
- Nagase H, Woessner JF (July 1999). "Matrix metalloproteinases". The Journal of Biological Chemistry. 274 (31): 21491–4. doi:10.1074/jbc.274.31.21491. PMID 10419448.
- Fosang AJ, Neame PJ, Hardingham TE, Murphy G, Hamilton JA (August 1991). "Cleavage of cartilage proteoglycan between G1 and G2 domains by stromelysins". The Journal of Biological Chemistry. 266 (24): 15579–82. PMID 1874716.
- Sirum KL, Brinckerhoff CE (October 1989). "Cloning of the genes for human stromelysin and stromelysin 2: differential expression in rheumatoid synovial fibroblasts". Biochemistry. 28 (22): 8691–8. doi:10.1021/bi00448a004. PMID 2605216.
- Lichtinghagen R, Helmbrecht T, Arndt B, Böker KH (February 1995). "Expression pattern of matrix metalloproteinases in human liver". European Journal of Clinical Chemistry and Clinical Biochemistry. 33 (2): 65–71. CiteSeerX 10.1.1.633.9185. doi:10.1515/cclm.1995.33.2.65. PMID 7632822.
- Nguyen Q, Murphy G, Hughes CE, Mort JS, Roughley PJ (October 1993). "Matrix metalloproteinases cleave at two distinct sites on human cartilage link protein". The Biochemical Journal. 295 (Pt 2): 595–8. doi:10.1042/bj2950595. PMC 1134922. PMID 7694569.
- Conca W, Willmroth F (June 1994). "Human T lymphocytes express a member of the Matrix Metalloproteinase gene family". Arthritis and Rheumatism. 37 (6): 951–6. doi:10.1002/art.1780370626. PMID 8003069.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Gack S, Vallon R, Schaper J, Rüther U, Angel P (April 1994). "Phenotypic alterations in fos-transgenic mice correlate with changes in Fos/Jun-dependent collagenase type I expression. Regulation of mouse metalloproteinases by carcinogens, tumor promoters, cAMP, and Fos oncoprotein". The Journal of Biological Chemistry. 269 (14): 10363–9. PMID 8144618.
- Windsor LJ, Grenett H, Birkedal-Hansen B, Bodden MK, Engler JA, Birkedal-Hansen H (August 1993). "Cell type-specific regulation of SL-1 and SL-2 genes. Induction of the SL-2 gene but not the SL-1 gene by human keratinocytes in response to cytokines and phorbolesters". The Journal of Biological Chemistry. 268 (23): 17341–7. PMID 8349617.
- Knäuper V, Murphy G, Tschesche H (January 1996). "Activation of human neutrophil procollagenase by stromelysin 2". European Journal of Biochemistry / FEBS. 235 (1–2): 187–91. doi:10.1111/j.1432-1033.1996.00187.x. PMID 8631328.
- Pendás AM, Santamaría I, Alvarez MV, Pritchard M, López-Otín C (October 1996). "Fine physical mapping of the human matrix metalloproteinase genes clustered on chromosome 11q22.3". Genomics. 37 (2): 266–8. doi:10.1006/geno.1996.0557. PMID 8921407.
- Sorsa T, Salo T, Koivunen E, Tyynelä J, Konttinen YT, Bergmann U, Tuuttila A, Niemi E, Teronen O, Heikkilä P, Tschesche H, Leinonen J, Osman S, Stenman UH (August 1997). "Activation of type IV procollagenases by human tumor-associated trypsin-2". The Journal of Biological Chemistry. 272 (34): 21067–74. doi:10.1074/jbc.272.34.21067. PMID 9261109.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Madlener M, Werner S (November 1997). "cDNA cloning and expression of the gene encoding murine stromelysin-2 (MMP-10)". Gene. 202 (1–2): 75–81. doi:10.1016/S0378-1119(97)00456-3. PMID 9427548.
- Nakamura H, Fujii Y, Ohuchi E, Yamamoto E, Okada Y (April 1998). "Activation of the precursor of human stromelysin 2 and its interactions with other matrix metalloproteinases". European Journal of Biochemistry / FEBS. 253 (1): 67–75. doi:10.1046/j.1432-1327.1998.2530067.x. PMID 9578462.
- Bord S, Horner A, Hembry RM, Compston JE (July 1998). "Stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10) expression in developing human bone: potential roles in skeletal development". Bone. 23 (1): 7–12. doi:10.1016/S8756-3282(98)00064-7. PMID 9662124.
External links
- The MEROPS online database for peptidases and their inhibitors: M10.006
- Overview of all the structural information available in the PDB for UniProt: P09238 (Stromelysin-2) at the PDBe-KB.