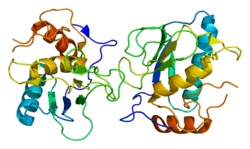Matrix metallopeptidase 13
Collagenase 3 is an enzyme that in humans is encoded by the MMP13 gene.[5][6] It is a member of the matrix metalloproteinase (MMP) family. Like most MMPs, it is secreted as an inactive pro-form. It is activated once the pro-domain is cleaved, leaving an active enzyme composed of the catalytic domain and the hemopexin-like domain PDB: 1PEX. Although the actual mechanism has not been described, the hemopexin domain participates in collagen degradation, the catalytic domain alone being particularly inefficient in collagen degradation. During embryonic development, MMP13 is expressed in the skeleton as required for restructuring the collagen matrix for bone mineralization. In pathological situations it is highly overexpressed; this occurs in human carcinomas, rheumatoid arthritis and osteoarthritis.[7]
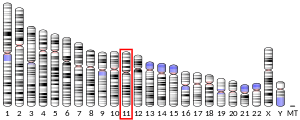
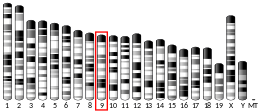
Proteins of the matrix metalloproteinase (MMP) family are involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, and tissue remodeling, as well as in disease processes, such as arthritis and metastasis. Most MMPs are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. The protein encoded by this gene cleaves type II collagen more efficiently than types I and III. It may be involved in articular cartilage turnover and cartilage pathophysiology associated with osteoarthritis. The gene is part of a cluster of MMP genes which localize to chromosome 11q22.3.[6]
Regulation
Transcriptional regulation of MMP-13 is tightly controlled due to its potent proteolytic capacity. There are several binding domains for various transcription factors including AP-1, PEA-3 and OSE-2 as well as a sequence with homology to a TGF-β inhibitory element (TIE). Moreover, several cytokines and growth factors have been demonstrated to affect Mmp13 gene expression, including parathyroid hormone, IGF-1, TGF-β, hepatocyte growth factor and many inflammatory cytokines such as IL-1α and IL-1β.[8]
The upstream regulatory region of the Mmp13 gene contains a number of transcription factor binding sites but it was recently discovered that there is a conserved forkhead response element (FHRE) consensus sequence for FOXO3a in the human, mouse and rat Mmp13 promoter. Endogenous FOXO3a activation results in marked upregulation of Mmp13 expression which is capable of promoting extracellular matrix degradation and apoptotic cell death. [9]
Clinical Relevance
MMP-13 has long been a protein of interest in the context of osteoarthritis and rheumatoid arthritis.[10]
The role of MMP-13 has also been thoroughly examined in atherosclerosis, specifically in potentially reducing the collagen content of the fibrous cap. [11] [12] [13] [14] [15]
References
- GRCh38: Ensembl release 89: ENSG00000137745 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000050578 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Freije JM, Díez-Itza I, Balbín M, Sánchez LM, Blasco R, Tolivia J, López-Otín C (June 1994). "Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas". The Journal of Biological Chemistry. 269 (24): 16766–73. PMID 8207000.
- "Entrez Gene: MMP13 matrix metallopeptidase 13 (collagenase 3)".
- Johansson N, Ahonen M, Kähäri VM (January 2000). "Matrix metalloproteinases in tumor invasion". Cellular and Molecular Life Sciences. 57 (1): 5–15. doi:10.1007/s000180050495. PMID 10949577.
- Leeman MF, Curran S, Murray GI (2003). "The structure, regulation, and function of human matrix metalloproteinase-13". Critical Reviews in Biochemistry and Molecular Biology. 37 (3): 149–66. doi:10.1080/10409230290771483. PMID 12139441.
- Yu H, Fellows A, Foote K, Yang Z, Figg N, Littlewood T, Bennett M (March 2018). "FOXO3a (Forkhead Transcription Factor O Subfamily Member 3a) Links Vascular Smooth Muscle Cell Apoptosis, Matrix Breakdown, Atherosclerosis, and Vascular Remodeling Through a Novel Pathway Involving MMP13 (Matrix Metalloproteinase 13)". Arteriosclerosis, Thrombosis, and Vascular Biology. 38 (3): 555–565. doi:10.1161/ATVBAHA.117.310502. PMID 29326312.
- Takaishi H, Kimura T, Dalal S, Okada Y, D'Armiento J (February 2008). "Joint diseases and matrix metalloproteinases: a role for MMP-13". Current Pharmaceutical Biotechnology. 9 (1): 47–54. doi:10.2174/138920108783497659. PMID 18289056.
- Sukhova GK, Schönbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, Libby P (May 1999). "Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques". Circulation. 99 (19): 2503–9. doi:10.1161/01.cir.99.19.2503. PMID 10330380.
- Deguchi JO, Aikawa E, Libby P, Vachon JR, Inada M, Krane SM, Whittaker P, Aikawa M (October 2005). "MMP-13/collagenase-3 deletion promotes collagen accumulation and organization in mouse atherosclerotic plaques". Circulation. 112 (17): 2708–2715.
- Cheng C, Tempel D, van Haperen R, van Damme L, Algür M, Krams R, de Crom R (May 2009). "Activation of MMP8 and MMP13 by angiotensin II correlates to severe intra-plaque hemorrhages and collagen breakdown in atherosclerotic lesions with a vulnerable phenotype". Atherosclerosis. 204 (1): 26–33. doi:10.1016/j.atherosclerosis.2009.01.025. PMID 19233360.
- Quillard T, Tesmenitsky Y, Croce K, Travers R, Shvartz E, Koskinas KC, et al. (November 2011). "Selective inhibition of matrix metalloproteinase-13 increases collagen content of established mouse atherosclerosis". Arteriosclerosis, Thrombosis, and Vascular Biology. 31 (11): 2464–72. doi:10.1161/ATVBAHA.111.231563. PMID 21903941.
- Quillard T, Araújo HA, Franck G, Tesmenitsky Y, Libby P (June 2014). "Matrix metalloproteinase-13 predominates over matrix metalloproteinase-8 as the functional interstitial collagenase in mouse atheromata". Arteriosclerosis, Thrombosis, and Vascular Biology. 34 (6): 1179–86. doi:10.1161/ATVBAHA.114.303326. PMID 24723558.
Further reading
- Nagase H, Woessner JF (July 1999). "Matrix metalloproteinases". The Journal of Biological Chemistry. 274 (31): 21491–4. doi:10.1074/jbc.274.31.21491. PMID 10419448.
- Pendás AM, Matilla T, Estivill X, López-Otín C (April 1995). "The human collagenase-3 (CLG3) gene is located on chromosome 11q22.3 clustered to other members of the matrix metalloproteinase gene family". Genomics. 26 (3): 615–8. doi:10.1016/0888-7543(95)80186-P. PMID 7607691.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, et al. (February 1996). "Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage". The Journal of Clinical Investigation. 97 (3): 761–8. doi:10.1172/JCI118475. PMC 507114. PMID 8609233.
- Knäuper V, Will H, López-Otin C, Smith B, Atkinson SJ, Stanton H, et al. (July 1996). "Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme". The Journal of Biological Chemistry. 271 (29): 17124–31. doi:10.1074/jbc.271.29.17124. PMID 8663255.
- Gomis-Rüth FX, Gohlke U, Betz M, Knäuper V, Murphy G, López-Otín C, Bode W (December 1996). "The helping hand of collagenase-3 (MMP-13): 2.7 A crystal structure of its C-terminal haemopexin-like domain". Journal of Molecular Biology. 264 (3): 556–66. doi:10.1006/jmbi.1996.0661. PMID 8969305.
- Knäuper V, Cowell S, Smith B, López-Otin C, O'Shea M, Morris H, et al. (March 1997). "The role of the C-terminal domain of human collagenase-3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction". The Journal of Biological Chemistry. 272 (12): 7608–16. doi:10.1074/jbc.272.12.7608. PMID 9065415.
- Pendás AM, Balbín M, Llano E, Jiménez MG, López-Otín C (March 1997). "Structural analysis and promoter characterization of the human collagenase-3 gene (MMP13)". Genomics. 40 (2): 222–33. doi:10.1006/geno.1996.4554. PMID 9119388.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Willmroth F, Peter HH, Conca W (February 1998). "A matrix metalloproteinase gene expressed in human T lymphocytes is identical with collagenase 3 from breast carcinomas". Immunobiology. 198 (4): 375–84. doi:10.1016/s0171-2985(98)80046-6. PMID 9562863.
- Lovejoy B, Welch AR, Carr S, Luong C, Broka C, Hendricks RT, et al. (March 1999). "Crystal structures of MMP-1 and -13 reveal the structural basis for selectivity of collagenase inhibitors". Nature Structural Biology. 6 (3): 217–21. doi:10.1038/6657. PMID 10074939.
- Barmina OY, Walling HW, Fiacco GJ, Freije JM, López-Otín C, Jeffrey JJ, Partridge NC (October 1999). "Collagenase-3 binds to a specific receptor and requires the low density lipoprotein receptor-related protein for internalization". The Journal of Biological Chemistry. 274 (42): 30087–93. doi:10.1074/jbc.274.42.30087. PMID 10514495.
- Lauer-Fields JL, Tuzinski KA, Shimokawa K, Nagase H, Fields GB (May 2000). "Hydrolysis of triple-helical collagen peptide models by matrix metalloproteinases". The Journal of Biological Chemistry. 275 (18): 13282–90. doi:10.1074/jbc.275.18.13282. PMID 10788434.
- Hiller O, Lichte A, Oberpichler A, Kocourek A, Tschesche H (October 2000). "Matrix metalloproteinases collagenase-2, macrophage elastase, collagenase-3, and membrane type 1-matrix metalloproteinase impair clotting by degradation of fibrinogen and factor XII". The Journal of Biological Chemistry. 275 (42): 33008–13. doi:10.1074/jbc.M001836200. PMID 10930399.
- McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM (August 2000). "Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3". Science. 289 (5482): 1202–6. Bibcode:2000Sci...289.1202M. doi:10.1126/science.289.5482.1202. PMID 10947989.
- Terp GE, Christensen IT, Jørgensen FS (June 2000). "Structural differences of matrix metalloproteinases. Homology modeling and energy minimization of enzyme-substrate complexes". Journal of Biomolecular Structure & Dynamics. 17 (6): 933–46. doi:10.1080/07391102.2000.10506582. PMID 10949161.
- Nakamura H, Fujii Y, Inoki I, Sugimoto K, Tanzawa K, Matsuki H, et al. (December 2000). "Brevican is degraded by matrix metalloproteinases and aggrecanase-1 (ADAMTS4) at different sites". The Journal of Biological Chemistry. 275 (49): 38885–90. doi:10.1074/jbc.M003875200. PMID 10986281.