MK-4482
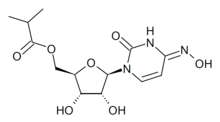
MK-4482, commonly known as EIDD-2801 until July 2020, is an experimental antiviral drug which is orally active (can be taken orally) and was developed for the treatment of influenza. It is a prodrug of the synthetic nucleoside derivative N4-hydroxycytidine, and exerts its antiviral action through introduction of copying errors during viral RNA replication.[1][2] Activity has also been demonstrated against coronaviruses including SARS, MERS and SARS-CoV-2.[3]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C13H19N3O7 |
| Molar mass | 329.31 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
The drug was developed at Emory University by the university's drug innovation company, DRIVE. It was then acquired by Miami-based company Ridgeback Biotherapeutics, who later partnered with Merck & Co. to develop the drug further. It was renamed by Merck in July 2020.
COVID-19
After being found to be active against SARS-CoV-2 in March 2020, MK-4482 was tested in a preliminary human study for "Safety, Tolerability, and Pharmacokinetics" in healthy volunteers in the UK and US.[6] In June 2020, Ridgeback Biotherapeutics announced it was moving to Phase II trials to test the efficacy of the drug as a treatment for COVID-19.[7]. Two trials of small numbers of hospitalized and non-hospitalized patients in the US and the UK were underway in July.[8][9] In late July 2020, and without yet releasing any medical data, Merck, which had been partnering with Ridgeback Biotherapeutics on developing the drug, announced its intention to move MK-4482 to late stage trials beginning in September 2020.[10]
References
- Toots M, Yoon JJ, Cox RM, Hart M, Sticher ZM, Makhsous N, et al. (October 2019). "Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia". Science Translational Medicine. 11 (515): eaax5866. doi:10.1126/scitranslmed.aax5866. PMC 6848974. PMID 31645453.
- Toots M, Yoon JJ, Hart M, Natchus MG, Painter GR, Plemper RK (April 2020). "Quantitative efficacy paradigms of the influenza clinical drug candidate EIDD-2801 in the ferret model". Translational Research. 218: 16–28. doi:10.1016/j.trsl.2019.12.002. PMID 31945316.
- Sheahan TP, Sims AC, Zhou S, Graham RL, Pruijssers AJ, Agostini ML, et al. (April 2020). "An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice". Science Translational Medicine. 12 (541): eabb5883. doi:10.1126/scitranslmed.abb5883. PMC 7164393. PMID 32253226.
- Halford, Bethany. "An emerging antiviral takes aim at COVID-19". Retrieved 1 August 2020.
- Cohen, Jon; Piller, Charles (13 May 2020). "Emails offer look into whistleblower charges of cronyism behind potential COVID-19 drug". Science. Retrieved 1 August 2020.
- "COVID-19 First In Human Study to Evaluate Safety, Tolerability, and Pharmacokinetics of EIDD-2801 in Healthy Volunteers". ClinicalTrials.gov. Retrieved 1 June 2020.
- "Ridgeback Biotherapeutics Announces Launch of Phase 2 Trials Testing EIDD-2801 as Potential Treatment for COVID-19". Business Wire. Retrieved 4 July 2020.
- "A Safety, Tolerability and Efficacy of EIDD-2801 to Eliminate Infectious Virus Detection in Persons With COVID-19". ClinicalTrials.gov. Retrieved 4 July 2020.
- "The Effect of EIDD-2801 on Viral Shedding of SARS-CoV-2 (COVID-19)". ClinicalTrials.gov. Retrieved 4 July 2020.
- Court, Emma (31 July 2020). "Merck pushes ahead on COVID-19 treatment, vaccines". Retrieved 31 July 2020.