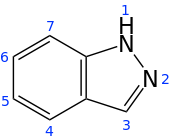
Indazole
Indazole, also called isoindazole, is a heterocyclic aromatic organic compound. This bicyclic compound consists of the fusion of benzene and pyrazole.
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1H-indazole | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.436 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H6N2 | |||
| Molar mass | 118.14 g/mol | ||
| Melting point | 147 to 149 °C (297 to 300 °F; 420 to 422 K) | ||
| Boiling point | 270 °C (518 °F; 543 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Indazole derivatives display a broad variety of biological activities.
Indazoles are rare in nature. The alkaloids nigellicine, nigeglanine, and nigellidine are indazoles. Nigellicine was isolated from the widely distributed plant Nigella sativa L. (black cumin). Nigeglanine was isolated from extracts of Nigella glandulifera.
The Davis–Beirut reaction can generate 2H-indazoles.
Indazole, C7H6N2, was obtained by E. Fischer (Ann. 1883, 221, p. 280) by heating ortho-hydrazine cinnamic acid,[1]
| C6H4 | CH = CH·COOH | =C2H4O2+C7H6N2. |
| NH·NH2 |
Some derivatives
- indazole-3-carboxylic acid
Having a carboxylic acid group on carbon 3. Can be further modified to lonidamine & tolnidamine.
gollark: It's much more maintainable due to not doing accursed things with unsafe raw memory access.
gollark: This is why you should use osmarkspythonbuildsystem™ instead.
gollark: Ah yes, -1-based indexing.
gollark: I've used lots of Haskell software which worked. Although it was hard to tell, since there were no side effects.
gollark: I have BACKDOORS into esobot but it isn't the same thing.
See also
- Indole, an analog with only one nitrogen atom in position 1.
- Benzimidazole, an analog with the nitrogen atoms in positions 1 and 3.
- Simple aromatic rings
- 7-Nitroindazole, an indazole-based nitric oxide synthase inhibitor
References
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 14 (11th ed.). Cambridge University Press. p. 371.
- Synthesis: W. Stadlbauer, in Science of Synthesis 2002, 12, 227, and W. Stadlbauer, in Houben-Weyl, 1994, E8b, 764.
- Review: A. Schmidt, A. Beutler, B. Snovydovych, Recent Advances in the Chemistry of Indazoles, Eur. J. Org. Chem. 2008, 4073 – 4095.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.