Pentazine
Pentazine is a hypothetical compound that consists of a six-membered aromatic ring containing five nitrogen atoms with the molecular formula CHN5. The name pentazine is used in the nomenclature of derivatives of this compound.
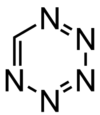 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| CHN5 | |
| Molar mass | 83.054 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pentazine is predicted to be unstable and to decompose into hydrogen cyanide (HCN) and nitrogen (N2).[1]
See also
- 6-membered rings with one nitrogen atom: pyridine
- 6-membered rings with two nitrogen atoms: diazines
- 6-membered rings with three nitrogen atoms: triazines
- 6-membered rings with four nitrogen atoms: tetrazines
- 6-membered rings with six nitrogen atoms: hexazine
References
- Hurst, Derek T. (1996). "Other Tetrazines and Pentazines". Comprehensive Heterocyclic Chemistry II. pp. 957–965. doi:10.1016/B978-008096518-5.00138-6. ISBN 9780080965185.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.