Hybrid-propellant rocket
A hybrid-propellant rocket is a rocket with a rocket motor that uses rocket propellants in two different phases: one solid and the other either gas or liquid. The hybrid rocket concept can be traced back to at least the 1930s.[1]

Hybrid rockets avoid some of the disadvantages of solid rockets like the dangers of propellant handling, while also avoiding some disadvantages of liquid rockets like their mechanical complexity.[2] Because it is difficult for the fuel and oxidizer to be mixed intimately (being different states of matter), hybrid rockets tend to fail more benignly than liquids or solids. Like liquid rocket engines, hybrid rocket motors can be shut down easily and the thrust is throttleable. The theoretical specific impulse () performance of hybrids is generally higher than solid motors and lower than liquid engines. as high as 400s has been measured in a hybrid rocket using metalized fuels.[3] Hybrid systems are more complex than solid ones, but they avoid significant hazards of manufacturing, shipping and handling solid rocket motors by storing the oxidizer and the fuel separately.
History
The first work on hybrid rockets was performed in the late 1930s at IG Farben in Germany and concurrently at the California Rocket Society in the United States. Leonid Andrussow, working in Germany, first theorized hybrid propellant rockets. O. Lutz, W. Noeggerath, and Andrussow tested a 10 kilonewtons (2,200 lbf) hybrid rocket motor using coal and gaseous N2O as the propellants. Oberth also worked on a hybrid rocket motor using LOX as the oxidizer and graphite as the fuel. The high heat of sublimation of carbon prevented these rocket motors from operating efficiently, as it resulted in a negligible burning rate.[4]

In the 1940s, the California Pacific Rocket Society used LOX in combination with several different fuel types, including wood, wax, and rubber. The most successful of these tests was with the rubber fuel, which is still the dominant fuel in use today. In June 1951, a LOX/rubber rocket was flown to an altitude of 9 kilometres (5.6 mi).[4]
Two major efforts occurred in the 1950s. One of these efforts was by G. Moore and K. Berman at General Electric. The duo used 90% H2O2 (high test peroxide, known as HTP) and polyethylene in a rod and tube grain design. They drew several significant conclusions from their work. The fuel grain had uniform burning. Grain cracks did not affect combustion, like it does with solid rocket motors. No hard starts were observed (a hard start is a pressure spike seen close to the time of ignition, typical of liquid rocket engines). The fuel surface acted as a flame holder, which encouraged stable combustion. The oxidizer could be throttled with one valve, and a high oxidizer to fuel ratio helped simplify combustion. The negative observations were low burning rates and that the thermal instability of peroxide was problematic for safety reasons. Another effort that occurred in the 1950s was development of a reverse hybrid. In a standard hybrid rocket motor, the solid material is the fuel. In a reverse hybrid rocket motor, the oxidizer is solid. William Avery of the Applied Physics Laboratory used jet fuel and ammonium nitrate, selected for their low cost. His O/F ratio was 0.035, which was 200 times smaller than the ratio used by Moore and Berman.[4]
In 1953 Pacific Rocket Society (est. 1943) was developing the XDF-23, a 4 inches (10 cm) x 72 inches (180 cm) hybrid rocket, designed by Jim Nuding, using LOX and rubber polymer called "Thiokol". They had already tried other fuels in prior iterations including cotton, paraffin wax and wood. The XDF name itself comes from "experimental Douglas fir" from one of the first units.[5]
In the 1960s, European organizations also began work on hybrid rockets. ONERA, based in France, and Volvo Flygmotor, based in Sweden, developed sounding rockets using hybrid rocket motor technology. The ONERA group focused on a hypergolic rocket motor, using nitric acid and an amine fuel. The company flew eight rockets: once in April 1964, three times in June 1965, and four times in 1967. The maximum altitude the flights achieved was over 100 kilometres (62 mi).[4] The Volvo Flygmotor group also used a hypergolic propellant combination. They also used nitric acid for their oxidizer, but used Tagaform (polybutadiene with an aromatic amine) as their fuel. Their flight was in 1969, lofting a 20 kilograms (44 lb) payload to 80 kilometres (50 mi).[4]
Meanwhile, in the United States, United Technologies Center (Chemical Systems Division) and Beech Aircraft were working on a supersonic target drone, known as Sandpiper. It used MON-25 (25% NO, 75% N2O4) as the oxidizer and polymethyl methacrylate (PMM)-Mg for the fuel. The drone flew six times in 1968, for more than 300 seconds and to an altitude greater than 160 kilometres (99 mi). The second iteration of the rocket, known as the HAST, had IRFNA-PB/PMM for its propellants and was throttleable over a 10/1 range. HAST could carry a heavier payload than the Sandpiper. Another iteration, which used the same propellant combination as the HAST, was developed by Chemical Systems Division and Teledyne Aircraft. Development for this program ended in the mid-1980s. Chemical Systems Division also worked on a propellant combination of lithium and FLOx (F2 and O2). This was an efficient hypergolic rocket that was throtteable. The vacuum specific impulse was 380 seconds at 93% combustion efficiency.[4]
AMROC developed the largest hybrid rockets ever created in the late 1980s and early 1990s. The first version of their engine, fired at the Air Force Phillips Laboratory, produced 312,000 newtons (70,000 lbf) of thrust for 70 seconds with a propellant combination of LOX and hydroxyl-terminated polybutadiene (HTPB). The second version of the motor, known as the H-250F, produced more than 1,000,000 newtons (220,000 lbf) of thrust.[4]
Korey Kline of Environmental Aeroscience Corporation (eAc) first fired a gaseous oxygen and rubber hybrid in 1982 at Lucerne Dry Lake, CA, after discussions on the technology with Bill Wood, formerly with Westinghouse.[6] The first SpaceShipOne hybrid tests were successfully conducted by Kline and eAc at Mojave, CA.[7]
In 1994, the U.S. Air Force Academy flew a hybrid sounding rocket to an altitude of 5 kilometres (3.1 mi). The 6.4 metres (21 ft) rocket used HTPB and LOX for its propellant, and reached a peak thrust of 4,400 newtons (990 lbf) and had a thrust duration of 16 seconds.[4]
Basic concepts
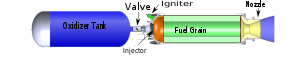
In its simplest form a hybrid rocket consists of a pressure vessel (tank) containing the liquid oxidiser, the combustion chamber containing the solid propellant, and a mechanical device separating the two. When thrust is desired, a suitable ignition source is introduced in the combustion chamber and the valve is opened. The liquid oxidiser (or gas) flows into the combustion chamber where it is vaporized and then reacted with the solid propellant. Combustion occurs in a boundary layer diffusion flame adjacent to the surface of the solid propellant.
Generally the liquid propellant is the oxidizer and the solid propellant is the fuel because solid oxidizers are extremely dangerous and lower performing than liquid oxidizers. Furthermore, using a solid fuel such as Hydroxyl-terminated polybutadiene (HTPB) or paraffin wax allows for the incorporation of high-energy fuel additives such as aluminium, lithium, or metal hydrides.
Combustion
The governing equation for hybrid rocket combustion shows that the regression rate is dependent on the oxidizer mass flux rate, which means the rate that the fuel will burn is proportional to the amount of oxidizer flowing through the port. This differs from a solid rocket motor, in which the regression rate is proportional to the chamber pressure of the motor.[4]
- where is the regression rate, ao is the regression rate coefficient (incorporating the grain length), Go is the oxidizer mass flux rate, and n is the regression rate exponent.[4]
As the motor burns, the increase in diameter of the fuel port results in an increased fuel mass flow rate. This phenomenon makes the oxidizer to fuel ratio (O/F) shift during the burn. The increased fuel mass flow rate can be compensated for by also increasing the oxidizer mass flow rate. In addition to the O/F varying as a function of time, it also varies based on the position down the fuel grain. The closer the position is to the top of the fuel grain, the higher the O/F ratio. Since the O/F varies down the port, a point called the stoichiometric point may exist at some point down the grain.[4]
Properties
Hybrid rocket motors exhibit some obvious as well as some subtle advantages over liquid-fuel rockets and solid-fuel rockets. A brief summary of some of these is given below:
Advantages compared with liquid rockets
- Mechanically simpler – requires only a single liquid propellant resulting in less plumbing, fewer valves, and simpler operations.
- Denser fuel – fuels in the solid phase generally have higher density than those in the liquid phase, reducing overall system volume.
- Metal additives – reactive metals such as aluminium, magnesium, lithium or beryllium can be easily included in the fuel grain increasing specific impulse(), density, or both.
- Combustion instabilities – Hybrid rockets do not typically exhibit high frequency combustion instabilities that plague liquid rockets due to the solid fuel grain breaking up acoustic waves that would otherwise reflect in an open liquid engine combustion chamber.
- Propellant pressurization – One of the most difficult to design portions of a liquid rocket system are the turbopumps. Turbopump design is complex as it has to precisely and efficiently pump and keep separated two fluids of different properties in precise ratios at very high volumetric flow rates, often cryogenic temperatures, and highly volatile chemicals while combusting those same fluids in order to power itself. Hybrids have far less fluid to move and can often be pressurized by a blow-down system (which would be prohibitively heavy in a liquid rocket) or self-pressurized oxidizers (such as N2O).
- Cooling – Liquid rockets often depend on one of the propellants, typically the fuel, to cool the combustion chamber and nozzle due to the very high heat fluxes and vulnerability of the metal walls to oxidation and stress cracking. Hybrid rockets have combustion chambers that are lined with the solid propellant which shields it from the product gases. Their nozzles are often graphite or coated in ablative materials similarly to solid rocket motors. The design, construction, and testing of liquid cooling flows is complex, making the system more prone to failure.
Advantages compared with solid rockets
- Higher theoretical – Possible due to limits of known solid oxidizers compared to often used liquid oxidizers.
- Less explosion hazard – Propellant grain is more tolerant of processing errors such as cracks since the burn rate is dependent on oxidizer mass flux rate. Propellant grain cannot be ignited by stray electrical charge and is very insensitive to auto-igniting due to heat. Hybrid rocket motors can be transported to the launch site with the oxidizer and fuel stored separately, improving safety.
- Fewer handling and storage issues – Ingredients in solid rockets are often incompatible chemically and thermally. Repeated changes in temperature can cause distortion of the grain. Antioxidants and coatings are used to keep the grain from breaking down or decomposing.
- More controllable – Stop/restart and throttling are all easily incorporated into most designs. Solid rockets rarely can be shut down easily and almost never have throttling or restart capabilities.
Disadvantages of hybrid rockets
Hybrid rockets also exhibit some disadvantages when compared with liquid and solid rockets. These include:
- Oxidizer-to-fuel ratio shift ("O/F shift") – with a constant oxidizer flow-rate, the ratio of fuel production rate to oxidizer flow rate will change as a grain regresses. This leads to off-peak operation from a chemical performance point of view. However, for a well-designed hybrid, O/F shift has a very small impact on performance because is insensitive to O/F shift near the peak.
- Low regression-rate (rate at which the solid phase recedes) fuels often drive multi-port fuel grains. Multi-port fuel grains have poor volumetric efficiency and, often, structural deficiencies. High regression rate liquefying fuels developed in the late 1990s offer a potential solution to this problem.[8]
- Compared with liquid-based propulsion, re-fueling a partially or totally depleted hybrid rocket would present significant challenges, as the solid propellant cannot simply be pumped into a fuel tank. This may or may not be an issue, depending upon how the rocket is planned to be used.
In general, much less development work has been completed with hybrids than liquids or solids and it is likely that some of these disadvantages could be rectified through further investment in research and development.
One problem in designing large hybrid orbital rockets is that turbopumps become necessary to achieve high flow rates and pressurization of the oxidizer. This turbopump must be powered by something. In a traditional liquid-propellant rocket, the turbopump uses the same fuel and oxidizer as the rocket, since they are both liquid and can be fed to the pre-burner. But in a hybrid, the fuel is solid and cannot be fed to a turbopump's engine. Some hybrids use an oxidizer that can also be used as a monopropellant, such as nitromethane or hydrogen peroxide, and so a turbopump can run on it alone. But nitromethane and hydrogen peroxide are significantly less efficient than liquid oxygen, which cannot be used alone to run a turbopump. Another fuel would be needed, requiring its own tank and decreasing rocket performance.
Fuel
Common fuel choices
A reverse hybrid rocket, which is not very common, is one where the engine uses a solid oxidizer and a liquid fuel. Some liquid fuel options are kerosene, hydrazine, and LH2. Common fuels for a typical hybrid rocket engine include polymers such as acrylic, polyethylene (PE), cross-linked rubber such as HTPB or liquefying fuels such as paraffin wax. Plexiglass was a common fuel, since the combustion could be visible through the transparent combustion chamber. HTPB is currently the most popular fuel for hybrid rocket engines, due to its energy and due to how safe it is to handle. Tests have been performed in which HTPB was soaked in liquid oxygen, and it still did not become explosive. These fuels are generally not as dense as solid rocket motors, so they are often doped with aluminum to increase the density and therefore the rocket performance.[4]:404
Grain manufacturing methods
Cast
Hybrid rocket fuel grains can be manufactured via casting techniques, since they are typically a plastic or a rubber. Complex geometries, which are driven by the need for higher fuel mass flow rates, makes casting fuel grains for hybrid rockets expensive and time-consuming due in part to equipment costs. On a larger scale, cast grains must be supported by internal webbing, so that large chunks of fuel do not impact or even potentially block the nozzle. Grain defects are also an issue in larger grains. Traditional fuels that are cast are hydroxyl-terminated polybutadiene (HTPB) and paraffin waxes.[9]
Additive manufacturing

Additive manufacturing is currently being used to create grain structures that were otherwise not possible to manufacture. Helical ports have been shown to increase fuel regression rates while also increasing volumetric efficiency.[10] An example of material used for a hybrid rocket fuel is acrylonitrile butadiene styrene (ABS). The printed material is also typically enhanced with additives to improve rocket performance.[9] Recent work at the University of Tennessee Knoxville has shown that, due to the increased surface area, the use of powdered fuels (i.e. graphite, coal, aluminum) encased in a 3D printed, ABS matrix can significantly increase the fuel burn rate and thrust level as compared to traditional polymer grains.[11]
Oxidizer
Common oxidizer choices
Common oxidizers include gaseous or liquid oxygen, nitrous oxide, and hydrogen peroxide. For a reverse hybrid, oxidizers such as frozen oxygen and ammonium perchlorate are used.[4]:405–406
Proper oxidizer vaporization is important for the rocket to perform efficiently. Improper vaporization can lead to very large regression rate differences at the head end of the motor when compared to the aft end. One method is to use a hot gas generator to heat the oxidizer in a pre-combustion chamber. Another method is to use an oxidizer that can also be used as a monopropellant. A good example is hydrogen peroxide, which can be catalytically decomposed over a silver bed into hot oxygen and steam. A third method is to inject a propellant that is hypergolic with the oxidizer into the flow. Some of the oxidizer will decompose, heating up the rest of the oxidizer in the flow.[4]:406–407
Hybrid safety
Generally, well designed and carefully constructed hybrids are very safe. The primary hazards associated with hybrids are:
- Pressure vessel failures – Chamber insulation failure may allow hot combustion gases near the chamber walls leading to a "burn-through" in which the vessel ruptures.
- Blow back – For oxidizers that decompose exothermically such as nitrous oxide or hydrogen peroxide, flame or hot gasses from the combustion chamber can propagate back through the injector, igniting the oxidizer and leading to a tank explosion. Blow-back requires gases to flow back through the injector due to insufficient pressure drop which can occur during periods of unstable combustion. Blow back is inherent to specific oxidizers and is not possible with oxidizers such as oxygen or nitrogen tetroxide unless fuel is present in the oxidizer tank.
- Hard starts – An excess of oxidizer in the combustion chamber prior to ignition, particularly for monopropellants such as nitrous oxide, can result in a temporary over-pressure or "spike" at ignition.
Because the fuel in a hybrid does not contain an oxidizer, it will not combust explosively on its own. For this reason, hybrids are classified as having no TNT equivalent explosive power. In contrast, solid rockets often have TNT equivalencies similar in magnitude to the mass of the propellant grain. Liquid-fuel rockets typically have TNT equivalencies calculated based on the amount of fuel and oxidizer which could realistically intimately combine before igniting explosively; this is often taken to be 10–20% of the total propellant mass. For hybrids, even filling the combustion chamber with oxidizer prior to ignition will not generally create an explosion with the solid fuel, the explosive equivalence is often quoted as 0%.
Organizations working on hybrids
Commercial companies
In 1998 SpaceDev acquired all of the intellectual property, designs, and test results generated by over 200 hybrid rocket motor firings by the American Rocket Company over its eight-year life. SpaceShipOne, the first private manned spacecraft, was powered by SpaceDev's hybrid rocket motor burning HTPB with nitrous oxide. However, nitrous oxide was the prime substance responsible for the explosion that killed three in the development of the successor of SpaceShipOne at Scaled Composites in 2007.[12][13] The Virgin Galactic SpaceShipTwo follow-on commercial suborbital spaceplane uses a scaled-up hybrid motor.
SpaceDev was developing the SpaceDev Streaker, an expendable small launch vehicle, and SpaceDev Dream Chaser, capable of both suborbital and orbital human space flight. Both Streaker and Dream Chaser use hybrid rocket motors that burn nitrous oxide and the synthetic rubber HTPB. SpaceDev was acquired by Sierra Nevada Corporation in 2009, becoming its Space Systems division, which continues to develop Dream Chaser for NASA's Commercial Crew Development contract. Sierra Nevada also developed RocketMotorTwo, the hybrid engine for SpaceShipTwo. On October 31, 2014 SpaceShipTwo was lost, initial speculation had suggested that its hybrid engine had in fact exploded and killed one test pilot and seriously injured the other. However investigation data now indicates an early deployment of the SpaceShip-Two feather system was the cause for aerodynamic breakup of the vehicle.[14]
U.S. Rockets[15] manufactured and deployed hybrids using self-pressurizing nitrous oxide N2O and HTPB as well as HTP and HTPB. The High Test Hydrogen Peroxide H2O2 86% and Hydroxyl-terminated polybutadiene (HTPB) and aluminum hybrids developed by U.S. Rockets produced a sea level delivered specific impulse (Isp) of 240, well above the typical 180 of N2O-HTPB hybrids. In addition to that, they were self-starting, restartable, had considerably lower combustion instability making them suitable for fragile or manned missions such as Bloodhound SSC, SpaceShip Two or SpaceShip Three. The company had successfully tested[16] and deployed both pressure fed and pump fed versions of the latter HTP-HTPB style. Deliverables to date have ranged from 6-inch to 18-inch diameter, and developed units up to 54-inch diameter. The vendor claimed scalability to over 5 meters diameter with regression rates approaching solids, according to literature distributed at the November 2013 Defense Advanced Research Projects Agency meeting for XS-1. U.S Rockets is not longer manufacturing large scale rockets.[17]
Gilmour Space Technologies began testing Hybrid rocket engines in 2015 with both N2O and HP with HDPE and HDPE wax blends. 2016 testing includes a 5000 Lb HP/PE engine. The company is planning to use hybrids for both sounding and orbital rockets.
Orbital Technologies Corporation (Orbitec) has been involved in some US government-funded research on hybrid rockets including the "Vortex Hybrid" concept.[18]
Environmental Aeroscience Corporation (eAc)[19] was incorporated in 1994 to develop hybrid rocket propulsion systems. It was included in the design competition for the SpaceShipOne motor but lost the contract to SpaceDev. Environmental Aeroscience Corporation still supplied parts to SpaceDev for the oxidizer fill, vent, and dump system.[20]
Rocket Crafters Inc. (RCI) builds and tests hybrid rockets in Cocoa, Florida. They have conducted 40+ subscale tests of their STAR-3D engine, and conducted tests of a 5000 lbf test engine at their facility in Cocoa. They use liquid Nitrous Oxide in combination with a 3D printed ABS plastic fuel grain. They plan their first suborbital flight out of Spaceport New Mexico in the Summer of 2020. [21]
Rocket Lab sells hybrid sounding rockets and related technology.
The Reaction Research Society (RRS), although known primarily for their work with liquid rocket propulsion, has a long history of research and development with hybrid rocket propulsion.
Copenhagen Suborbitals, a Danish rocket group, has designed and test-fired several hybrids using N2O at first and currently LOX. Their fuel is epoxy, paraffin wax, or polyurethane.[22] The group eventually moved away from hybrids because of thrust instabilities, and now uses a motor similar to that of the V-2 rocket.
TiSPACE is a Taiwanese company which is developing a family of hybrid-propellant rockets.[23]
Universities
Space Propulsion Group was founded in 1999 by Dr. Arif Karabeyoglu, Prof. Brian Cantwell and others from Stanford University to develop high regression-rate liquefying hybrid rocket fuels. They have successfully fired motors as large as 12.5 in. diameter which produce 13,000 lbf. using the technology and are currently developing a 24 in. diameter, 25,000 lbf. motor to be initially fired in 2010. Stanford University is the institution where liquid-layer combustion theory for hybrid rockets was developed. The SPaSE group at Stanford is currently working with NASA Ames Research Center developing the Peregrine Sounding rocket which will be capable of 100 km altitude.[24] Engineering challenges include various types of combustion instabilities.[25] Although the proposed motor was test fired in 2013, the Peregrine program eventually switched to a standard solid rocket for its 2016 debut.
The University of Tennessee Knoxville has carried out hybrid rocket research since 1999, working in collaboration with NASA Marshall Space Flight Center and private industry. This work has included the integration of a water-cooled calorimeter nozzle, one of the first 3D-printed, hot section components successfully used in a rocket motor.[26] Other work at the university has focused on the use of bio-derived fuels[27] and powdered fuels encased in a 3D-printed, ABS matrix.
At the Delft University of Technology, the student team Delft Aerospace Rocket Engineering (DARE) is very active in the design and building of hybrid rockets. In October 2015, DARE broke the European student altitude record with the Stratos II+ sounding rocket. Stratos II+ was propelled by the DHX-200 hybrid rocket engine, using a nitrous oxide oxidizer and fuel blend of paraffin, sorbitol and aluminium powder. On July 26, 2018 DARE attempted to launch the Stratos III hybrid rocket. This rocket used the same fuel/oxidizer combination as its predecessor, but with an increased impulse of around 400 kNs. Unfortunately, the vehicle was lost 20 seconds into the flight.[28]
Florida Institute of Technology has successfully tested and evaluated hybrid technologies with their Panther Project. The WARR[29] student-team at the Technical University of Munich has been developing hybrid engines and rockets since the early 1970s. Using acids, oxygen or nitrous oxide in combination with polyethylene or HTPB. The development includes test stand engines as well as airborne versions, like the first German hybrid rocket Barbarella. They are currently working on a hybrid rocket with Liquid oxygen as its oxidizer, to break the European height record of amateur rockets. They are also working with Rocket Crafters and testing their hybrid rockets.
Boston University's student-run "Rocket Propulsion Group",[30] which in the past has launched only solid motor rockets, is attempting to design and build a single-stage hybrid sounding rocket to launch into sub-orbital space by July 2015.[31]
Brigham Young University (BYU), the University of Utah, and Utah State University launched a student-designed rocket called Unity IV in 1995 which burned the solid fuel hydroxyl-terminated polybutadiene (HTPB) with an oxidizer of gaseous oxygen, and in 2003 launched a larger version which burned HTPB with nitrous oxide.
University of Brasilia's Hybrid Team has extensive research in paraffin wax/N2 hybrids having already made more than 50 tests fires. Hybrid Team is currently working liquefied propellant, numeric optimization and rocket design. Nowadays the rocket design team, called Capital Rocket Team, is developing high power hybrid rockets and researching about some additives. The Chemical Propulsion Laboratory has already made some researches and is developing the motor for SARA platform.
University of California, Los Angeles's student-run "University Rocket Project" launches hybrid propulsion rockets utilizing Nitrous Oxide as an oxidizer and HTPB as the fuel. They are currently in the development process of their third student-built hybrid rocket engine.
University of Toronto's student-run "University of Toronto Aerospace Team", designs and builds hybrid engine powered rockets. They are currently constructing a new engine testing facility at the University of Toronto Institute for Aerospace Studies, and are working towards breaking the Canadian amateur rocketry altitude record with their new rocket, Defiance MKIII, currently under rigorous testing. Defiance MKIII's engine, QUASAR, is a Nitrous-Paraffin hybrid engine, capable of producing 7 kN of thrust for a period of 9 seconds.
In 2016, Pakistan's DHA Suffa University successfully developed[32] Raheel-1, hybrid rocket engines in 1 kN class, using paraffin wax and liquid oxygen, thereby becoming the first university run rocket research program in the country.[33] In India, Birla Institute of Technology, Mesra Space engineering and rocketry department has been working on Hybrid Projects with various fuels and oxidizers.
Pars Rocketry Group from Istanbul Technical University has designed and built the first hybrid rocket engine of Turkey, the rocket engine extensively tested in May 2015.[34]
A United Kingdom-based team (laffin-gas) is using four N2O hybrid rockets in a drag-racing style car. Each rocket has an outer diameter of 150mm and is 1.4m long. They use a fuel grain of high-density wound paper soaked in cooking oil. The N2O supply is provided by Nitrogen-pressurised piston accumulators which provide a higher rate of delivery than N2O gas alone and also provide damping of any reverse shock.
In Italy one of the leading centers for research in hybrid propellants rockets is CISAS (Center of Studies and Activities for Space) "G. Colombo", University of Padua. The activities cover all stages of the development: from theoretical analysis of the combustion process to numerical simulation using CFD codes, and then by conducting ground tests of small scale and large-scale rockets (up to 20 kN, N2O-Paraffin wax based motors). One of these engines flew successfully in 2009. Since 2014, the research group is focused on the use of High-Test Peroxide as oxidizer, in partnership with "Technology for Propulsion and Innovation", a university of Padua spin-off company.[35]
In Taiwan, hybrid rocket system developments began in 2009 through R&D projects of NSPO with two university teams. Both teams employed nitrous oxide/HTPB propellant system with different improvement schemes. One team (NCKU) added 50 percent of paraffin in the solid grain for boosting the regression rates. The other team (ARRC/NCTU) incorporated innovative mixing enhancement devices to push the overall combustion efficiency towards the theoretical value. This team takes full advantage of high-fidelity simulations and experimental works for very cost-effective developments. Several hybrid rockets have been successfully launched so far, reaching altitudes of 10–20 km. Their plans include attempting 100–200 km altitude launch to test nanosatellites by the end of 2014, and developing orbital launch capabilities for nanosatellites in the long run. A sub-scale N2O/PE Dual-Vortical-Flow (DVF) hybrid engine hot-fire test in 2014 has delivered an averaged sea-level Isp of 280 sec, which indicates that the system has reached around 97% combustion efficiency.
In (Germany) the University of Stuttgart's Student team HyEnd is the current world record holder for the highest flying student built hybrid rocket with their Heros.[36]
Many other universities, such as Embry-Riddle Aeronautical University, the University of Washington, Purdue University, the University of Michigan at Ann Arbor, the University of Arkansas at Little Rock, Hendrix College, the University of Illinois, Portland State University, University of KwaZulu-Natal, Texas A&M University, Aarhus University, Rice University and AGH University of Science and Technology have hybrid motor test stands that allow for student research with hybrid rockets.
High power rocketry
There are a number of hybrid rocket motor systems available for amateur/hobbyist use in high-powered model rocketry. These include the popular HyperTek systems[37] and a number of 'Urbanski-Colburn Valved' (U/C) systems such as RATTWorks,[38] Contrail Rockets,[39] and Propulsion Polymers.[40] All of these systems use nitrous oxide as the oxidizer and a plastic fuel (such as Polyvinyl chloride(PVC) or Polypropylene) or a polymer-based fuel such as HTPB. This reduces the cost per flight compared to solid rocket motors, although there is generally more 'GSE' (ground support equipment) required with hybrids.
In popular culture
An October 26, 2005 episode of the Television show MythBusters entitled "Confederate Rocket" [41] featured a hybrid rocket motor using liquid nitrous oxide and paraffin wax. The myth purported that during the American Civil War, the Confederate Army was able to construct a rocket of this type. The myth was revisited in a later episode entitled Salami Rocket, using hollowed out dry salami as the solid fuel.
In the February 18, 2007, episode of Top Gear, a Reliant Robin was used by Richard Hammond and James May in an attempt to modify a normal K-reg Robin into a reusable space shuttle. Steve Holland, a professional radio-controlled aircraft pilot, helped Hammond to work out how to land a Robin safely. The craft was built by senior members of the United Kingdom Rocketry Association (UKRA) and achieved a successful launch, flew for several seconds into the air and managed to successfully jettison the solid-fuel rocket boosters on time. This was the largest rocket launched by a non-government organisation in Europe. It used 6 x 40960 NS O Contrail Rockets motors giving a maximum thrust of 8 tonnes. However, the car failed to separate from the large external fuel tank due to faulty explosive bolts between the Robin and the external tank, and the Robin subsequently crashed into the ground and seemed to have exploded soon after. This explosion was added for dramatic effect as neither Reliant Robins nor hybrid rocket motors explode in the way depicted.
See also
References
- "GIRD-09". Encyclopedia Astronautix. Retrieved June 25, 2017.
- "Hybrid Rocket Propulsion Overview". Space Propulsion Group, Inc.
- "A Brief History of Hybrid Rocket Technology". Space Propulsion Group, Inc. Archived from the original on July 16, 2011. Retrieved October 15, 2010.
- Humble, Ronald; Gary, Henry; Larson, Wiley (1995). Space Propulsion Analysis and Design. McGraw-Hill. ISBN 978-0-07-031320-0.
- Shep Shepherd (April 1954). "With the amateur – but serious – rocketeers out on the Mojave desert, it's Fourth of July the year around". Popular Mechanics. Hearst Magazines. pp. 81–85.
- "This is how LMR and HPR got started..." California Rocketry magazine.
- eAc photo gallery Gallery of photos from the first successful SpaceShipOne static test with Korey Kline of eAc and Burt Rutan of Scaled Composites.
- "Wax Hybrids". Science@NASA. Archived from the original on May 23, 2009. Retrieved June 1, 2009.
- "Hybrid Rocket Engines Use Additive Manufacturing to Combine the Advantages of Solid and Liquid Propellants". Stratasys. Retrieved December 19, 2016.
- Walker, Sean (2015). "High Regression Rate Hybrid Rocket Fuel Grains with Helical Port Structures": 40. Bibcode:2016PhDT.........6W. Cite journal requires
|journal=(help) - "The Use of a 3-D Printed, Polymer Matrix Containing Pulverized Fuel in a Hybrid Rocket," J.E. Lyne et al, The University of Tennessee, 2018 Joint Propulsion Conference.
- Bosker, Bianca (November 30, 2009). "Virgin Galactic SpaceShipTwo getting ready for test flights ahead of space tourism". HuffPost.
- Dorneanu, Lucian. "Spaceship Explosion at the Mojave Desert Test Area Kills 2".
- "Virgin Galactic's SpaceShipTwo Crashes: 1 Dead, 1 Injured – NBC News".
- "Archived copy". Archived from the original on January 2, 2014. Retrieved January 2, 2014.CS1 maint: archived copy as title (link)
- Video of an 18" diameter self-starting and ending HTP-HTPB hybrid near Garlock, CA. , October 17, 2009. Retrieved December 31, 2013.
- https://www.facebook.com/pg/US-Rockets-219639027743/posts/?ref=page_internal
- Orbitec Orbitec Vortex Hybrid Test, with photo. Retrieved April 23, 2016.
- EAC Company home page. . Retrieved October 4, 2017.
- http://www.hybrids.com/tier1.html
- http://www.rocketcrafters.com/rocket-crafters-concludes-comet-testing/
- Copenhagen Suborbitals Archived May 27, 2010, at the Wayback Machine HEAT booster development and tests, with photos and video. Retrieved June 3, 2010
- Chia-nan, Lin. "FEATURE: Firm sets sights on heavens as space industry develops". www.taipeitimes.com. Retrieved February 17, 2020.
- "Peregrine rocket poster (2008)" (PDF). Archived from the original (PDF) on February 27, 2009.. Stanford University
- "Peregrine rocket poster (2012)" (PDF). Archived from the original (PDF) on April 13, 2014.. Stanford University
- "Development of a Three-Dimensional Printed, Liquid-Cooled Nozzle for a Hybrid Rocket," Nick Quigley and J.E. Lyne, The University of Tennessee at Knoxville, Journal of Propulsion and Power, Nov.–Dec. 2014.
- Putnam, Scott Grayson, "Investigation of Non-Conventional Bio-Derived Fuels for Hybrid Rocket Motors. " PhD diss., University of Tennessee, 2007. https://trace.tennessee.edu/utk_graddiss/269
- https://dare.tudelft.nl/2018/07/stratos-iii-launch-summary/
- "Raketentechnik". warr.de. Archived from the original on December 6, 2011. Retrieved June 27, 2011.
- "Rocket Propulsion Group", Boston University
- "Rocket Propulsion Group >> Starscraper" Archived January 3, 2015, at the Wayback Machine Boston University
- , First Hybrid Rocket Engine of Pakistan – YouTube
- , Pakistan's first-ever hybrid rocket readying for launch – The Express Tribune
- "ITU24 – Pars Rocketry Team", Istanbul Technical University
- "Hybrid Propellant | T4i - Space technology for innovation".
- "HEROS Launches".
- "HyperTEK – The Easiest Access of Them All". hypertekhybrids.com.
- "RATTworks: Precision Hybrid & Tribrid Rocket Motors". rattworks.net.
- "Contrail Rockets Hybrid Rocket Motors". contrailrockets.com.
- "初売りで流行のアイテムを入手しよう|人気のおしゃれグッズ". propulsionpolymers.com.
- https://go.discovery.com/tv-shows/mythbusters/videos/confederate-rocket
Further reading
- Costa, Fernando de Souza; Vieira, Ricardo (2010), "Preliminary analysis of hybrid rockets for launching nanosats into LEO", Journal of the Brazilian Society of Mechanical Sciences and Engineering, 32 (4): 502–509, doi:10.1590/S1678-58782010000400012
External links
| Wikimedia Commons has media related to Hybrid rocket engines. |