Hemocyanin
Hemocyanins (also spelled haemocyanins and abbreviated Hc) are proteins that transport oxygen throughout the bodies of some invertebrate animals. These metalloproteins contain two copper atoms that reversibly bind a single oxygen molecule (O2). They are second only to hemoglobin in frequency of use as an oxygen transport molecule. Unlike the hemoglobin in red blood cells found in vertebrates, hemocyanins are not bound to blood cells but are instead suspended directly in the hemolymph. Oxygenation causes a color change between the colorless Cu(I) deoxygenated form and the blue Cu(II) oxygenated form.[1]
| Hemocyanin, copper containing domain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
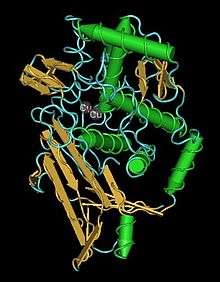 Single oxygenated functional unit from the hemocyanin of an octopus | |||||||||||
| Identifiers | |||||||||||
| Symbol | Hemocyanin_M | ||||||||||
| Pfam | PF00372 | ||||||||||
| InterPro | IPR000896 | ||||||||||
| PROSITE | PDOC00184 | ||||||||||
| SCOPe | 1lla / SUPFAM | ||||||||||
| |||||||||||
| Hemocyanin, all-alpha domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
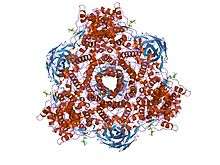 Crystal structure of hexameric haemocyanin from Panulirus interruptus refined at 3.2 angstroms resolution | |||||||||
| Identifiers | |||||||||
| Symbol | Hemocyanin_N | ||||||||
| Pfam | PF03722 | ||||||||
| InterPro | IPR005204 | ||||||||
| PROSITE | PDOC00184 | ||||||||
| SCOPe | 1lla / SUPFAM | ||||||||
| |||||||||
| Hemocyanin, ig-like domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
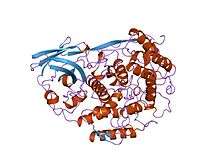 crystallographic analysis of oxygenated and deoxygenated states of arthropod hemocyanin shows unusual differences | |||||||||
| Identifiers | |||||||||
| Symbol | Hemocyanin_C | ||||||||
| Pfam | PF03723 | ||||||||
| InterPro | IPR005203 | ||||||||
| PROSITE | PDOC00184 | ||||||||
| SCOPe | 1lla / SUPFAM | ||||||||
| |||||||||
Species distribution
Hemocyanins are found only in the Mollusca and Arthropoda: the earliest discoveries of hemocyanins were in the snail Helix pomatia (a mollusc) and in the horseshoe crab (an arthropod). They were subsequently found to be common among cephalopods and crustaceans and are utilized by some land arthropods such as the tarantula Eurypelma californicum,[2] the emperor scorpion,[3] and the centipede Scutigera coleoptrata. Also, larval storage proteins in many insects appear to be derived from hemocyanins.[4]
The hemocyanin superfamily
The arthropod hemocyanin superfamily is composed of phenoloxidases, hexamerins, pseudohemocyanins or cryptocyanins, (dipteran) hexamerin receptors.[5]
Phenoloxidase are copper containing tyrosinases. These proteins are involved in the process of sclerotization of arthropod cuticle, in wound healing, and humoral immune defense. Phenoloxidase is synthesized by zymogens and are activated by cleaving a N-terminal peptide.
Hexamerins are storage proteins commonly found in insects. These proteins are synthesized by the larval fat body and are associated with molting cycles or nutritional conditions.
Pseudohemocyanin and cryptocyanins genetic sequences are closely related to hemocyanins in crustaceans. These proteins have a similar structure and function, but lack the copper binding sites.
The evolutionary changes within the phylogeny of the hemocyanin superfamily are closely related to the emergence of these different proteins in various species. The understanding of proteins within this superfamily would not be well understood without the extensive studies of hemocyanin in arthropods.[6]
Structure and mechanism

Although the respiratory function of hemocyanin is similar to that of hemoglobin, there are a significant number of differences in its molecular structure and mechanism. Whereas hemoglobin carries its iron atoms in porphyrin rings (heme groups), the copper atoms of hemocyanin are bound as prosthetic groups coordinated by histidine residues. The active site of hemocyanin is composed of a pair of copper(I) cations which are directly coordinated to the protein through the driving force of imidazolic rings of six histidine residues.[7] It has been noted that species using hemocyanin for oxygen transportation include crustaceans living in cold environments with low oxygen pressure. Under these circumstances hemoglobin oxygen transportation is less efficient than hemocyanin oxygen transportation.[8] Nevertheless, there are also terrestrial arthropods using hemocyanin, notably spiders and scorpions, that live in warm climates. The molecule is conformationally stable and fully functioning at temperatures up to 90 degrees C.[9]
Most hemocyanins bind with oxygen non-cooperatively and are roughly one-fourth as efficient as hemoglobin at transporting oxygen per amount of blood. Hemoglobin binds oxygen cooperatively due to steric conformation changes in the protein complex, which increases hemoglobin's affinity for oxygen when partially oxygenated. In some hemocyanins of horseshoe crabs and some other species of arthropods, cooperative binding is observed, with Hill coefficients of 1.6–3.0. Hill coefficients vary depending on species and laboratory measurement settings. Hemoglobin, for comparison, has a Hill coefficient of usually 2.8–3.0. In these cases of cooperative binding hemocyanin was arranged in protein sub-complexes of 6 subunits (hexamer) each with one oxygen binding site; binding of oxygen on one unit in the complex would increase the affinity of the neighboring units. Each hexamer complex was arranged together to form a larger complex of dozens of hexamers. In one study, cooperative binding was found to be dependent on hexamers being arranged together in the larger complex, suggesting cooperative binding between hexamers. Hemocyanin oxygen-binding profile is also affected by dissolved salt ion levels and pH.[10]
Hemocyanin is made of many individual subunit proteins, each of which contains two copper atoms and can bind one oxygen molecule (O2). Each subunit weighs about 75 kilodaltons (kDa). Subunits may be arranged in dimers or hexamers depending on species; the dimer or hexamer complex is likewise arranged in chains or clusters with weights exceeding 1500 kDa. The subunits are usually homogeneous, or heterogeneous with two variant subunit types. Because of the large size of hemocyanin, it is usually found free-floating in the blood, unlike hemoglobin.[11]
Hexamers are characteristic of arthropod hemocyanins.[12] A hemocyanin of the tarantula Eurypelma californicum[2] is made up of 4 hexamers or 24 peptide chains. A hemocyanin from the house centipede Scutigera coleoptrata[13] is made up of 6 hexamers or 36 chains. Horseshoe crabs have an 8-hexamer (i. e. 48-chain) hemocyanin. Simple hexamers are found in the spiny lobster Panulirus interruptus and the isopod Bathynomus giganteus.[12] Peptide chains in crustaceans are about 660 amino acid residues long, and in chelicerates they are about 625. In the large complexes there is a variety of variant chains, all about the same length; pure components do not usually self-assemble.
Catalytic activity
Hemocyanin is homologous to the phenol oxidases (e.g. tyrosinase) since both proteins share type 3 Cu active site coordination.[14] In both cases inactive proenzymes such as hemocyanin, tyrosinase, and catcholoxidase must be activated first. This is done by removing the amino acid that blocks the entrance channel to the active site when the proenzyme is not active. There is currently no other known modifications necessary to activate the proenzyme and enable catalytic activity. Conformational differences determine the type of catalytic activity that the hemocyanin is able to perform.[15] Hemocyanin also exhibits phenol oxidase activity, but with slowed kinetics from greater steric bulk at the active site. Partial denaturation actually improves hemocyanin’s phenol oxidase activity by providing greater access to the active site.[1][14]
Spectral properties
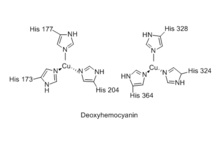
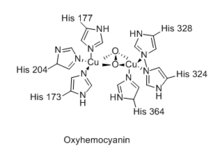
Spectroscopy of oxyhemocyanin shows several salient features:[16]
- Resonance Raman spectroscopy shows that O2 is bound in a symmetric environment (ν(O-O) is not IR-allowed).
- OxyHc is EPR-silent indicating the absence of unpaired electrons
- Infrared spectroscopy shows ν(O-O) of 755 cm−1
Much work has been devoted to preparing synthetic analogues of the active site of hemocyanin.[16] One such model, which features a pair of copper centers bridged side-on by peroxo ligand, shows ν(O-O) at 741 cm−1 and a UV-Vis spectrum with absorbances at 349 and 551 nm. Both of these measurements agree with the experimental observations for oxyHc.[17] The Cu-Cu separation in the model complex is 3.56 Å, that of oxyhemocyanin is ca. 3.6 Å (deoxyHc: ca. 4.6 Å).[17][18][19]
Anticancer effects
The hemocyanin found in the blood of the Chilean abalone, Concholepas concholepas, has immunotherapeutic effects against bladder cancer in murine models. Mice primed with C. concholepas before implantation of bladder tumor (MBT-2) cells. Mice treated with C. concholepas hemocyanin showed antitumor effects: prolonged survival, decreased tumor growth and incidence, and lack of toxic effects and may have a potential use in future immunotherapy for superficial bladder cancer.[20]
Keyhole limpet hemocyanin (KLH) is an immune stimulant derived from circulating glycoproteins of the marine mollusk Megathura crenulata. KLH has been shown to be a significant treatment against the proliferations of breast cancer, pancreas cancer, and prostate cancer cells when delivered in vitro. Keyhole limpet hemocyanin inhibits growth of human Barrett's esophageal cancer through both apoptic and nonapoptic mechanisms of cell death.[21]
Case studies: environmental impact on hemocyanin levels
A 2003 study of the effect of culture conditions of blood metabolites and hemocyanin of the white shrimp Litopenaeus vannamei found that the levels of hemocyanin, oxyhemocyanin in particular, are affected by the diet. The study compared oxyhemocyanin levels in the blood of white shrimp housed in an indoor pond with a commercial diet with that of white shrimp housed in an outdoor pond with a more readily available protein source (natural live food) as well. Oxyhemocyanin and blood glucose levels were higher in shrimp housed in outdoor ponds. It was also found that blood metabolite levels tended to be lower in low activity level species, such as crabs, lobsters, and the indoor shrimp when compared to the outdoor shrimp. This correlation is possibly indicative of the morphological and physiological evolution of crustaceans. The levels of these blood proteins and metabolites appear to be dependent on energetic demands and availability of those energy sources.[22]
References
- Coates CJ, Nairn J (July 2014). "Diverse immune functions of hemocyanins". Developmental and Comparative Immunology. 45 (1): 43–55. doi:10.1016/j.dci.2014.01.021. PMID 24486681.
- Voit R, Feldmaier-Fuchs G, Schweikardt T, Decker H, Burmester T (December 2000). "Complete sequence of the 24-mer hemocyanin of the tarantula Eurypelma californicum. Structure and intramolecular evolution of the subunits". The Journal of Biological Chemistry. 275 (50): 39339–44. doi:10.1074/jbc.M005442200. PMID 10961996.
- Jaenicke E, Pairet B, Hartmann H, Decker H (2012). "Crystallization and preliminary analysis of crystals of the 24-meric hemocyanin of the emperor scorpion (Pandinus imperator)". PLOS One. 7 (3): e32548. Bibcode:2012PLoSO...732548J. doi:10.1371/journal.pone.0032548. PMC 3293826. PMID 22403673. Lay summary – Johannes Gutenberg-Universität Mainz (June 22, 2012).
- Beintema JJ, Stam WT, Hazes B, Smidt MP (1994). "Evolution of arthropod hemocyanins and insect storage proteins (hexamerins)". Mol Biol Evol. 11 (3): 493–503. doi:10.1093/oxfordjournals.molbev.a040129. PMID 8015442.
- Burmester, T (Feb 2002). "Origin and evolution of arthropod hemocyanins and related proteins". Journal of Comparative Physiology B. 172 (2): 95–107. doi:10.1007/s00360-001-0247-7. PMID 11916114.
- Burmester T (February 2001). "Molecular evolution of the arthropod hemocyanin superfamily". Molecular Biology and Evolution. 18 (2): 184–95. doi:10.1093/oxfordjournals.molbev.a003792. PMID 11158377.
- Rannulu NS, Rodgers MT (March 2005). "Solvation of copper ions by imidazole: structures and sequential binding energies of Cu+(imidazole)x, x = 1-4. Competition between ion solvation and hydrogen bonding". Physical Chemistry Chemical Physics. 7 (5): 1014–25. Bibcode:2005PCCP....7.1014R. doi:10.1039/b418141g. PMID 19791394.
- Strobel A, Hu MY, Gutowska MA, Lieb B, Lucassen M, Melzner F, Pörtner HO, Mark FC (December 2012). "Influence of temperature, hypercapnia, and development on the relative expression of different hemocyanin isoforms in the common cuttlefish Sepia officinalis" (PDF). Journal of Experimental Zoology Part A. 317 (8): 511–23. doi:10.1002/jez.1743. PMID 22791630.
- Sterner R, Vogl T, Hinz HJ, Penz F, Hoff R, Föll R, Decker H (1995). "Extreme thermostability of tarantula hemocyanin". FEBS Lett. 364: 9–12. doi:10.1016/0014-5793(95)00341-6. PMID 7750550.
- Perton FG, Beintema JJ, Decker H (May 1997). "Influence of antibody binding on oxygen binding behavior of Panulirus interruptus hemocyanin". FEBS Letters. 408 (2): 124–6. doi:10.1016/S0014-5793(97)00269-X. PMID 9187351.
- Waxman L (May 1975). "The structure of arthropod and mollusc hemocyanins". The Journal of Biological Chemistry. 250 (10): 3796–806. PMID 1126935.
- van Holde KE, Miller KI (1995). Anfinsen CB, Richards FM, Edsall JT, Eisenberg DS (eds.). Hemocyanins. Advances in Protein Chemistry. 47. pp. 1–81. doi:10.1016/S0065-3233(08)60545-8. ISBN 978-0-12-034247-1. PMID 8561049.
- Kusche K, Hembach A, Hagner-Holler S, Gebauer W, Burmester T (July 2003). "Complete subunit sequences, structure and evolution of the 6 x 6-mer hemocyanin from the common house centipede, Scutigera coleoptrata". European Journal of Biochemistry. 270 (13): 2860–8. doi:10.1046/j.1432-1033.2003.03664.x. PMID 12823556.
- Decker H, Tuczek F (August 2000). "Tyrosinase/catecholoxidase activity of hemocyanins: structural basis and molecular mechanism" (PDF). Trends in Biochemical Sciences. 25 (8): 392–7. doi:10.1016/S0968-0004(00)01602-9. PMID 10916160.
- Decker H, Schweikardt T, Nillius D, Salzbrunn U, Jaenicke E, Tuczek F (August 2007). "Similar enzyme activation and catalysis in hemocyanins and tyrosinases". Gene. 398 (1–2): 183–91. doi:10.1016/j.gene.2007.02.051. PMID 17566671.
- Elwell, Courtney E.; Gagnon, Nicole L.; Neisen, Benjamin D.; Dhar, Debanjan; Spaeth, Andrew D.; Yee, Gereon M.; Tolman, William B. (2017). "Copper–Oxygen Complexes Revisited: Structures, Spectroscopy, and Reactivity". Chemical Reviews. 117 (3): 2059–2107. doi:10.1021/acs.chemrev.6b00636. PMC 5963733. PMID 28103018.
- Kitajima N, Fujisawa K, Fujimoto C, Morooka Y, Hashimoto S, Kitagawa T, Toriumi K, Tatsumi K, Nakamura A (1992). "A new model for dioxygen binding in hemocyanin. Synthesis, characterization, and molecular structure of the μ-η2:η2 peroxo dinuclear copper(II) complexes, [Cu(BH(3,5-R2pz)3)]2(O2) (R = i-Pr and Ph)". Journal of the American Chemical Society. 114 (4): 1277–91. doi:10.1021/ja00030a025.
- Gaykema WP, Hol WG, Vereijken JM, Soeter NM, Bak HJ, Beintema JJ (1984). "3.2 Å structure of the copper-containing, oxygen-carrying protein Panulirus interruptus haemocyanin". Nature. 309 (5963): 23–9. Bibcode:1984Natur.309...23G. doi:10.1038/309023a0.
- Kodera M, Katayama K, Tachi Y, Kano K, Hirota S, Fujinami S, Suzuki M (1999). "Crystal Structure and Reversible O2-Binding of a Room Temperature Stable μ-η2:η2-Peroxodicopper(II) Complex of a Sterically Hindered Hexapyridine Dinucleating Ligand". Journal of the American Chemical Society. 121 (47): 11006–7. doi:10.1021/ja992295q.
- Atala A (2006). "This Month in Investigative Urology". The Journal of Urology. 176 (6): 2335–6. doi:10.1016/j.juro.2006.09.002.
- McFadden DW, Riggs DR, Jackson BJ, Vona-Davis L (November 2003). "Keyhole limpet hemocyanin, a novel immune stimulant with promising anticancer activity in Barrett's esophageal adenocarcinoma". American Journal of Surgery. 186 (5): 552–5. doi:10.1016/j.amjsurg.2003.08.002. PMID 14599624.
- Pascual C, Gaxiola G, Rosas C (2003). "Blood metabolites and hemocyanin of the white shrimp, Litopenaeus vannamei: The effect of culture conditions and a comparison with other crustacean species". Marine Biology. 142 (4): 735. doi:10.1007/s00227-002-0995-2.
Further reading
- Rehm P, Pick C, Borner J, Markl J, Burmester T (February 2012). "The diversity and evolution of chelicerate hemocyanins". BMC Evolutionary Biology. 12: 19. doi:10.1186/1471-2148-12-19. PMC 3306762. PMID 22333134.
- Ali SA, Abbasi A (2011). Scorpion Hemocyanin: The blue blood. Saarbrücken: VDM Verlag Dr. Müller. p. 160. ISBN 978-3-639-33725-9.
External links
| Wikimedia Commons has media related to Hemocyanin. |
- 3D hemocyanin structures in the EM Data Bank (EMDB)
- Overview of all the structural information available in the PDB for UniProt: P04253 (Hemocyanin II) at the PDBe-KB.