Fermentation
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In the context of food production, it may more broadly refer to any process in which the activity of microorganisms brings about a desirable change to a foodstuff or beverage.[1] The science of fermentation is known as zymology.

In microorganisms, fermentation is the primary means of producing adenosine triphosphate (ATP) by the degradation of organic nutrients anaerobically.[2] Humans have used fermentation to produce foodstuffs and beverages since the Neolithic age. For example, fermentation is used for preservation in a process that produces lactic acid found in such sour foods as pickled cucumbers, kombucha, kimchi, and yogurt, as well as for producing alcoholic beverages such as wine and beer. Fermentation also occurs within the gastrointestinal tracts of all animals, including humans.[3]
Definitions
Below are some definitions of fermentation. They range from informal, general usages to more scientific definitions.[4]
- Preservation methods for food via microorganisms (general use).
- Any large-scale microbial process occurring with or without air (common definition used in industry).
- Any process that produces alcoholic beverages or acidic dairy products (general use).
- Any energy-releasing metabolic process that takes place only under anaerobic conditions (somewhat scientific).
- Any metabolic process that releases energy from a sugar or other organic molecule, does not require oxygen or an electron transport system, and uses an organic molecule as the final electron acceptor (most scientific).
Biological role
Along with photosynthesis and aerobic respiration, fermentation is a way of extracting energy from molecules, but it is the only one common to all bacteria and eukaryotes. It is therefore considered the oldest metabolic pathway, suitable for an environment that did not yet have oxygen.[5]:389 Yeast, a form of fungus, occurs in almost any environment capable of supporting microbes, from the skins of fruits to the guts of insects and mammals and the deep ocean, and harvests sugar-rich materials to produce ethanol and carbon dioxide.[6][7]
The basic mechanism for fermentation remains present in all cells of higher organisms. Mammalian muscle carries out fermentation during periods of intense exercise where oxygen supply becomes limited, resulting in the creation of lactic acid.[8]:63 In invertebrates, fermentation also produces succinate and alanine.[9]:141
Fermentative bacteria play an essential role in the production of methane in habitats ranging from the rumens of cattle to sewage digesters and freshwater sediments. They produce hydrogen, carbon dioxide, formate and acetate and carboxylic acids; and then consortia of microbes convert the carbon dioxide and acetate to methane. Acetogenic bacteria oxidize the acids, obtaining more acetate and either hydrogen or formate. Finally, methanogens (in the domain Archea) convert acetate to methane.[10]
Biochemical overview

Fermentation reacts NADH with an endogenous, organic electron acceptor.[2] Usually this is pyruvate formed from sugar through glycolysis. The reaction produces NAD+ and an organic product, typical examples being ethanol, lactic acid, and hydrogen gas (H2), and often also carbon dioxide. However, more exotic compounds can be produced by fermentation, such as butyric acid and acetone. Fermentation products are considered waste products, since they cannot be metabolized further without the use of oxygen.[12]
Fermentation normally occurs in an anaerobic environment. In the presence of O2, NADH, and pyruvate are used to generate ATP in respiration. This is called oxidative phosphorylation, and it generates much more ATP than glycolysis alone since it releases the chemical energy of O2.[12] For that reason, fermentation is rarely utilized when oxygen is available. However, even in the presence of abundant oxygen, some strains of yeast such as Saccharomyces cerevisiae prefer fermentation to aerobic respiration as long as there is an adequate supply of sugars (a phenomenon known as the Crabtree effect).[13] Some fermentation processes involve obligate anaerobes, which cannot tolerate oxygen.
Although yeast carries out the fermentation in the production of ethanol in beers, wines, and other alcoholic drinks, this is not the only possible agent: bacteria carry out the fermentation in the production of xanthan gum.
Products
Ethanol
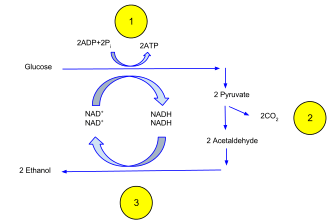
In ethanol fermentation, one glucose molecule is converted into two ethanol molecules and two carbon dioxide molecules.[14][15] It is used to make bread dough rise: the carbon dioxide forms bubbles, expanding the dough into a foam.[16][17] The ethanol is the intoxicating agent in alcoholic beverages such as wine, beer and liquor.[18] Fermentation of feedstocks, including sugarcane, corn, and sugar beets, produces ethanol that is added to gasoline.[19] In some species of fish, including goldfish and carp, it provides energy when oxygen is scarce (along with lactic acid fermentation).[20]
The figure illustrates the process. Before fermentation, a glucose molecule breaks down into two pyruvate molecules (Glycolysis). The energy from this exothermic reaction is used to bind inorganic phosphates to ADP, which converts it to ATP, and convert NAD+ to NADH. The pyruvates break down into two acetaldehyde molecules and give off two carbon dioxide molecules as waste products. The acetaldehyde is reduced into ethanol using the energy and hydrogen from NADH, and the NADH is oxidized into NAD+ so that the cycle may repeat. The reaction is catalyzed by the enzymes pyruvate decarboxylase and alcohol dehydrogenase.[14]
Lactic acid
Homolactic fermentation (producing only lactic acid) is the simplest type of fermentation. Pyruvate from glycolysis[21] undergoes a simple redox reaction, forming lactic acid.[22][23] It is probably the only respiration process that does not produce a gas as a byproduct. Overall, one molecule of glucose (or any six-carbon sugar) is converted to two molecules of lactic acid:
- C6H12O6 → 2 CH3CHOHCOOH
It occurs in the muscles of animals when they need energy faster than the blood can supply oxygen. It also occurs in some kinds of bacteria (such as lactobacilli) and some fungi. It is the type of bacteria that convert lactose into lactic acid in yogurt, giving it its sour taste. These lactic acid bacteria can carry out either homolactic fermentation, where the end-product is mostly lactic acid, or heterolactic fermentation, where some lactate is further metabolized to ethanol and carbon dioxide[22] (via the phosphoketolase pathway), acetate, or other metabolic products, e.g.:
- C6H12O6 → CH3CHOHCOOH + C2H5OH + CO2
If lactose is fermented (as in yogurts and cheeses), it is first converted into glucose and galactose (both six-carbon sugars with the same atomic formula):
- C12H22O11 + H2O → 2 C6H12O6
Heterolactic fermentation is in a sense intermediate between lactic acid fermentation and other types, e.g. alcoholic fermentation. Reasons to go further and convert lactic acid into something else include:
- The acidity of lactic acid impedes biological processes. This can be beneficial to the fermenting organism as it drives out competitors that are unadapted to the acidity. As a result, the food will have a longer shelf life (one reason foods are purposely fermented in the first place); however, beyond a certain point, the acidity starts affecting the organism that produces it.
- The high concentration of lactic acid (the final product of fermentation) drives the equilibrium backwards (Le Chatelier's principle), decreasing the rate at which fermentation can occur and slowing down growth.
- Ethanol, into which lactic acid can be easily converted, is volatile and will readily escape, allowing the reaction to proceed easily. CO2 is also produced, but it is only weakly acidic and even more volatile than ethanol.
- Acetic acid (another conversion product) is acidic and not as volatile as ethanol; however, in the presence of limited oxygen, its creation from lactic acid releases additional energy. It is a lighter molecule than lactic acid, forming fewer hydrogen bonds with its surroundings (due to having fewer groups that can form such bonds), thus is more volatile and will also allow the reaction to proceed more quickly.
- If propionic acid, butyric acid, and longer monocarboxylic acids are produced (see mixed acid fermentation), the amount of acidity produced per glucose consumed will decrease, as with ethanol, allowing faster growth.
Hydrogen gas
Hydrogen gas is produced in many types of fermentation as a way to regenerate NAD+ from NADH. Electrons are transferred to ferredoxin, which in turn is oxidized by hydrogenase, producing H2.[14] Hydrogen gas is a substrate for methanogens and sulfate reducers, which keep the concentration of hydrogen low and favor the production of such an energy-rich compound,[24] but hydrogen gas at a fairly high concentration can nevertheless be formed, as in flatus.
For example, Clostridium pasteurianum ferments glucose to butyrate, acetate, carbon dioxide, and hydrogen gas:[25] The reaction leading to acetate is:
- C6H12O6 + 4 H2O → 2 CH3COO− + 2 HCO3− + 4 H+ + 4 H2
Other
Other types of fermentation include mixed acid fermentation, butanediol fermentation, butyrate fermentation, caproate fermentation, acetone–butanol–ethanol fermentation, and glyoxylate fermentation.
Modes of operation
Most industrial fermentation uses batch or fed-batch procedures, although continuous fermentation can be more economical if various challenges, particularly the difficulty of maintaining sterility, can be met.[26]
Batch
In a batch process, all the ingredients are combined and the reactions proceed without any further input. Batch fermentation has been used for millennia to make bread and alcoholic beverages, and it is still a common method, especially when the process is not well understood.[27]:1 However, it can be expensive because the fermentor must be sterilized using high pressure steam between batches.[26] Strictly speaking, there is often addition of small quantities of chemicals to control the pH or suppress foaming.[27]:25
Batch fermentation goes through a series of phases. There is a lag phase in which cells adjust to their environment; then a phase in which exponential growth occurs. Once many of the nutrients have been consumed, the growth slows and becomes non-exponential, but production of secondary metabolites (including commercially important antibiotics and enzymes) accelerates. This continues through a stationary phase after most of the nutrients have been consumed, and then the cells die.[27]:25
Fed-batch
Fed-batch fermentation is a variation of batch fermentation where some of the ingredients are added during the fermentation. This allows greater control over the stages of the process. In particular, production of secondary metabolites can be increased by adding a limited quantity of nutrients during the non-exponential growth phase. Fed-batch operations are often sandwiched between batch operations.[27]:1[28]
Open
The high cost of sterilizing the fermentor between batches can be avoided using various open fermentation approaches that are able to resist contamination. One is to use a naturally evolved mixed culture. This is particularly favored in wastewater treatment, since mixed populations can adapt to a wide variety of wastes. Thermophilic bacteria can produce lactic acid at temperatures of around 50 °Celsius, sufficient to discourage microbial contamination; and ethanol has been produced at a temperature of 70 °C. This is just below its boiling point (78 °C), making it easy to extract. Halophilic bacteria can produce bioplastics in hypersaline conditions. Solid-state fermentation adds a small amount of water to a solid substrate; it is widely used in the food industry to produce flavors, enzymes and organic acids.[26]
Continuous
In continuous fermentation, substrates are added and final products removed continuously.[26] There are three varieties: chemostats, which hold nutrient levels constant; turbidostats, which keep cell mass constant; and plug flow reactors in which the culture medium flows steadily through a tube while the cells are recycled from the outlet to the inlet.[28] If the process works well, there is a steady flow of feed and effluent and the costs of repeatedly setting up a batch are avoided. Also, it can prolong the exponential growth phase and avoid byproducts that inhibit the reactions by continuously removing them. However, it is difficult to maintain a steady state and avoid contamination, and the design tends to be complex.[26] Typically the fermentor must run for over 500 hours to be more economical than batch processors.[28]
History of the use of fermentation
The use of fermentation, particularly for beverages, has existed since the Neolithic and has been documented dating from 7000–6600 BCE in Jiahu, China,[29] 5000 BCE in India, Ayurveda mentions many Medicated Wines, 6000 BCE in Georgia,[30] 3150 BCE in ancient Egypt,[31] 3000 BCE in Babylon,[32] 2000 BCE in pre-Hispanic Mexico,[32] and 1500 BC in Sudan.[33] Fermented foods have a religious significance in Judaism and Christianity. The Baltic god Rugutis was worshiped as the agent of fermentation.[34][35]

In 1837, Charles Cagniard de la Tour, Theodor Schwann and Friedrich Traugott Kützing independently published papers concluding, as a result of microscopic investigations, that yeast is a living organism that reproduces by budding.[36][37]:6 Schwann boiled grape juice to kill the yeast and found that no fermentation would occur until new yeast was added. However, a lot of chemists, including Antoine Lavoisier, continued to view fermentation as a simple chemical reaction and rejected the notion that living organisms could be involved. This was seen as a reversion to vitalism and was lampooned in an anonymous publication by Justus von Liebig and Friedrich Wöhler.[5]:108–109
The turning point came when Louis Pasteur (1822–1895), during the 1850s and 1860s, repeated Schwann's experiments and showed that fermentation is initiated by living organisms in a series of investigations.[23][37]:6 In 1857, Pasteur showed that lactic acid fermentation is caused by living organisms.[38] In 1860, he demonstrated that bacteria cause souring in milk, a process formerly thought to be merely a chemical change, and his work in identifying the role of microorganisms in food spoilage led to the process of pasteurization.[39] In 1877, working to improve the French brewing industry, Pasteur published his famous paper on fermentation, "Etudes sur la Bière", which was translated into English in 1879 as "Studies on fermentation".[40] He defined fermentation (incorrectly) as "Life without air",[41] but correctly showed that specific types of microorganisms cause specific types of fermentations and specific end-products.
Although showing fermentation to be the result of the action of living microorganisms was a breakthrough, it did not explain the basic nature of the fermentation process, or prove that it is caused by the microorganisms that appear to be always present. Many scientists, including Pasteur, had unsuccessfully attempted to extract the fermentation enzyme from yeast.[41] Success came in 1897 when the German chemist Eduard Buechner ground up yeast, extracted a juice from them, then found to his amazement that this "dead" liquid would ferment a sugar solution, forming carbon dioxide and alcohol much like living yeasts.[42] Buechner's results are considered to mark the birth of biochemistry. The "unorganized ferments" behaved just like the organized ones. From that time on, the term enzyme came to be applied to all ferments. It was then understood that fermentation is caused by enzymes that are produced by microorganisms.[43] In 1907, Buechner won the Nobel Prize in chemistry for his work.[44]
Advances in microbiology and fermentation technology have continued steadily up until the present. For example, in the 1930s, it was discovered that microorganisms could be mutated with physical and chemical treatments to be higher-yielding, faster-growing, tolerant of less oxygen, and able to use a more concentrated medium.[45][46] Strain selection and hybridization developed as well, affecting most modern food fermentations.
Etymology
The word "ferment" is derived from the Latin verb fervere, which means to boil. It is thought to have been first used in the late 14th century in alchemy, but only in a broad sense. It was not used in the modern scientific sense until around 1600.
See also
| Wikisource has the text of the 1911 Encyclopædia Britannica article Fermentation. |
- List of fermented foods
- Acetone-butanol-ethanol fermentation
- Dark fermentation
- Fermentation in food processing
- Fermentation lock
- Gut fermentation syndrome
- Industrial fermentation
- Non-fermenter
- Photofermentation
- Aerobic fermentation
References
- Hui, Y. H. (2004). Handbook of vegetable preservation and processing. New York: M. Dekker. p. 180. ISBN 978-0-8247-4301-7. OCLC 52942889.
- Klein, Donald W.; Lansing M.; Harley, John (2006). Microbiology (6th ed.). New York: McGraw-Hill. ISBN 978-0-07-255678-0.
- Bowen, Richard. "Microbial Fermentation". Hypertexts for biological sciences. Colorado State University. Retrieved 29 April 2018.
- Tortora, Gerard J.; Funke, Berdell R.; Case, Christine L. (2010). "5". Microbiology An Introduction (10 ed.). San Francisco, CA: Pearson Benjamin Cummings. p. 135. ISBN 978-0-321-58202-7.
- Tobin, Allan; Dusheck, Jennie (2005). Asking about life (3rd ed.). Pacific Grove, Calif.: Brooks/Cole. ISBN 9780534406530.
- Martini, A. (1992). "Biodiversity and conservation of yeasts". Biodiversity and Conservation. 1 (4): 324–333. doi:10.1007/BF00693768. S2CID 35231385.
- Bass, D.; Howe, A.; Brown, N.; Barton, H.; Demidova, M.; Michelle, H.; Li, L.; Sanders, H.; Watkinson, S. C; Willcock, S.; Richards, T. A (22 December 2007). "Yeast forms dominate fungal diversity in the deep oceans". Proceedings of the Royal Society B: Biological Sciences. 274 (1629): 3069–3077. doi:10.1098/rspb.2007.1067. PMC 2293941. PMID 17939990.
- Voet, Donald; Voet, Judith G. (2010). Biochemistry (4th ed.). Wiley Global Education. ISBN 9781118139936.
- Broda, E (2014). The Evolution of the Bioenergetic Processes. Progress in Biophysics and Molecular Biology. 21. Elsevier. pp. 143–208. ISBN 9781483136134. PMID 4913287.
- Ferry, J G (September 1992). "Methane from acetate". Journal of Bacteriology. 174 (17): 5489–5495. doi:10.1128/jb.174.17.5489-5495.1992. PMC 206491. PMID 1512186.
- Stryer, Lubert (1995). Biochemistry (fourth ed.). New York - Basingstoke: W. H. Freeman and Company. ISBN 978-0716720096.
- Schmidt-Rohr, K (2020). "Oxygen Is the High-Energy Molecule Powering Complex Multicellular Life: Fundamental Corrections to Traditional Bioenergetics". ACS Omega. 5: 2221–2233. doi:10.1021/acsomega.9b03352. PMC 7016920. PMID 32064383.
- Piškur, Jure; Compagno, Concetta (2014). Molecular mechanisms in yeast carbon metabolism. Springer. p. 12. ISBN 9783642550133.
- Purves, William K.; Sadava, David E.; Orians, Gordon H.; Heller, H. Craig (2003). Life, the science of biology (7th ed.). Sunderland, Mass.: Sinauer Associates. pp. 139–140. ISBN 978-0-7167-9856-9.
- Stryer, Lubert (1975). Biochemistry. W. H. Freeman and Company. ISBN 978-0-7167-0174-3.
- Logan, BK; Distefano, S (1997). "Ethanol content of various foods and soft drinks and their potential for interference with a breath-alcohol test". Journal of Analytical Toxicology. 22 (3): 181–3. doi:10.1093/jat/22.3.181. PMID 9602932.
- "The Alcohol Content of Bread". Canadian Medical Association Journal. 16 (11): 1394–5. November 1926. PMC 1709087. PMID 20316063.
- "Alcohol". Drugs.com. Retrieved 26 April 2018.
- James Jacobs, Ag Economist. "Ethanol from Sugar". United States Department of Agriculture. Archived from the original on 2007-09-10. Retrieved 2007-09-04.
- van Waarde, Aren; Thillart, G. Van den; Verhagen, Maria (1993). "Ethanol Formation and pH-Regulation in Fish". Surviving Hypoxia. pp. 157–170. ISBN 978-0-8493-4226-4.
- Introductory Botany: plants, people, and the Environment. Berg, Linda R. Cengage Learning, 2007. ISBN 978-0-534-46669-5. p. 86
- AP Biology. Anestis, Mark. 2nd Edition. McGraw-Hill Professional. 2006. ISBN 978-0-07-147630-0. p. 61
- A dictionary of applied chemistry, Volume 3. Thorpe, Sir Thomas Edward. Longmans, Green and Co., 1922. p.159
- Madigan, Michael T.; Martinko, John M.; Parker, Jack (1996). Brock biology of microorganisms (8th ed.). Prentice Hall. ISBN 978-0-13-520875-5. Retrieved 2010-07-12.
- Thauer, R.K.; Jungermann, K.; Decker, K. (1977). "Energy conservation in chemotrophic anaerobic bacteria". Bacteriological Reviews. 41 (1): 100–80. doi:10.1128/MMBR.41.1.100-180.1977. ISSN 0005-3678. PMC 413997. PMID 860983.
- Li, Teng; Chen, Xiang-bin; Chen, Jin-chun; Wu, Qiong; Chen, Guo-Qiang (December 2014). "Open and continuous fermentation: Products, conditions and bioprocess economy". Biotechnology Journal. 9 (12): 1503–1511. doi:10.1002/biot.201400084. PMID 25476917.
- Cinar, Ali; Parulekar, Satish J.; Undey, Cenk; Birol, Gulnur (2003). Batch fermentation modeling, monitoring, and control. New York: Marcel Dekker. ISBN 9780203911358.
- Schmid, Rolf D.; Schmidt-Dannert, Claudia (2016). Biotechnology : an illustrated primer (Second ed.). John Wiley & Sons. p. 92. ISBN 9783527335152.
- McGovern, P. E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G. R.; Moreau, R. A.; Nunez, A.; Butrym, E. D.; Richards, M. P.; Wang, C. -S.; Cheng, G.; Zhao, Z.; Wang, C. (2004). "Fermented beverages of pre- and proto-historic China". Proceedings of the National Academy of Sciences. 101 (51): 17593–17598. Bibcode:2004PNAS..10117593M. doi:10.1073/pnas.0407921102. PMC 539767. PMID 15590771.
- Vouillamoz, J. F.; McGovern, P. E.; Ergul, A.; Söylemezoğlu, G. K.; Tevzadze, G.; Meredith, C. P.; Grando, M. S. (2006). "Genetic characterization and relationships of traditional grape cultivars from Transcaucasia and Anatolia". Plant Genetic Resources: Characterization and Utilization. 4 (2): 144–158. CiteSeerX 10.1.1.611.7102. doi:10.1079/PGR2006114.
- Cavalieri, D; McGovern P.E.; Hartl D.L.; Mortimer R.; Polsinelli M. (2003). "Evidence for S. cerevisiae fermentation in ancient wine" (PDF). Journal of Molecular Evolution. 57 Suppl 1: S226–32. Bibcode:2003JMolE..57S.226C. CiteSeerX 10.1.1.628.6396. doi:10.1007/s00239-003-0031-2. PMID 15008419. 15008419. Archived from the original (PDF) on December 9, 2006. Retrieved 2007-01-28.
- "Fermented fruits and vegetables. A global perspective". FAO Agricultural Services Bulletins - 134. Archived from the original on January 19, 2007. Retrieved 2007-01-28.
- Dirar, H., (1993), The Indigenous Fermented Foods of the Sudan: A Study in African Food and Nutrition, CAB International, UK
- "Gintaras Beresneviius. M. Strijkovskio Kronikos" lietuvi diev sraas". spauda.lt.
- Rūgutis. Mitologijos enciklopedija, 2 tomas. Vilnius. Vaga. 1999. 293 p.
- Shurtleff, William; Aoyagi, Akiko. "A Brief History of Fermentation, East and West". Soyinfo Center. Soyfoods Center, Lafayette, California. Retrieved 30 April 2018.
- Lengeler, Joseph W.; Drews, Gerhart; Schlegel, Hans Günter, eds. (1999). Biology of the prokaryotes. Stuttgart: Thieme [u.a.] ISBN 9783131084118.
- Accomplishments of Louis Pasteur Archived 2010-11-30 at the Wayback Machine. Fjcollazo.com (2005-12-30). Retrieved on 2011-01-04.
- HowStuffWorks "Louis Pasteur". Science.howstuffworks.com (2009-07-01). Retrieved on 2011-01-04.
- Louis Pasteur (1879) Studies on fermentation: The diseases of beer, their causes, and the means of preventing them. Macmillan Publishers.
- Modern History Sourcebook: Louis Pasteur (1822–1895): Physiological theory of fermentation, 1879. Translated by F. Faulkner, D.C. Robb.
- New beer in an old bottle: Eduard Buchner and the Growth of Biochemical Knowledge. Cornish-Bowden, Athel. Universitat de Valencia. 1997. ISBN 978-84-370-3328-0. p. 25.
- The enigma of ferment: from the philosopher's stone to the first biochemical Nobel prize. Lagerkvist, Ulf. World Scientific Publishers. 2005. ISBN 978-981-256-421-4. p. 7.
- A treasury of world science, Volume 1962, Part 1. Runes, Dagobert David. Philosophical Library Publishers. 1962. p. 109.
- Steinkraus, Keith (2018). Handbook of Indigenous Fermented Foods (Second ed.). CRC Press. ISBN 9781351442510.
- Wang, H. L.; Swain, E. W.; Hesseltine, C. W. (1980). "Phytase of molds used in oriental food fermentation". Journal of Food Science. 45 (5): 1262–1266. doi:10.1111/j.1365-2621.1980.tb06534.x.
External links
| Wikimedia Commons has media related to Fermentation. |
.svg.png)