Exercise
Exercise is any bodily activity that enhances or maintains physical fitness and overall health and wellness.[1]

It is performed for various reasons, to aid growth and improve strength, preventing aging, developing muscles and the cardiovascular system, honing athletic skills, weight loss or maintenance, improving health[2] and also for enjoyment. Many individuals choose to exercise outdoors where they can congregate in groups, socialize, and enhance well-being.[3]
In terms of health benefits, the amount of recommended exercise depends upon the goal, the type of exercise, and the age of the person. Even doing a small amount of exercise is healthier than doing none.[4]
Classification

Physical exercises are generally grouped into three types, depending on the overall effect they have on the human body:[5]
- Aerobic exercise is any physical activity that uses large muscle groups and causes the body to use more oxygen than it would while resting.[5] The goal of aerobic exercise is to increase cardiovascular endurance.[6] Examples of aerobic exercise include running, cycling, swimming, brisk walking, skipping rope, rowing, hiking, dancing, playing tennis, continuous training, and long distance running.[5]
- Anaerobic exercise, which includes strength and resistance training, can firm, strengthen, and increase muscle mass, as well as improve bone density, balance, and coordination.[5] Examples of strength exercises are push-ups, pull-ups, lunges, squats, bench press. Anaerobic exercise also includes weight training, functional training, eccentric training, interval training, sprinting, and high-intensity interval training which increase short-term muscle strength.[5][7]
- Flexibility exercises stretch and lengthen muscles.[5] Activities such as stretching help to improve joint flexibility and keep muscles limber.[5] The goal is to improve the range of motion which can reduce the chance of injury.[5][8]
Physical exercise can also include training that focuses on accuracy, agility, power, and speed.[9]
Types of exercise can also be classified as dynamic or static. 'Dynamic' exercises such as steady running, tend to produce a lowering of the diastolic blood pressure during exercise, due to the improved blood flow. Conversely, static exercise (such as weight-lifting) can cause the systolic pressure to rise significantly, albeit transiently, during the performance of the exercise.[10]
Health effects
| Type of adaptation | Endurance training effects |
Strength training effects |
Sources |
|---|---|---|---|
| Muscle hypertrophy | ↔ | ↑ ↑ ↑ | [11] |
| Muscle strength and power | ↔ ↓ | ↑ ↑ ↑ | [11] |
| Muscle fiber size | ↔ ↑ | ↑ ↑ ↑ | [11] |
| Myofibrillar protein synthesis | ↔ ↑ | ↑ ↑ ↑ | [11] |
| Neuromuscular adaptations | ↔ ↑ | ↑ ↑ ↑ | [11] |
| Anaerobic capacity | ↑ | ↑ ↑ | [11] |
| Lactate tolerance | ↑ ↑ | ↔ ↑ | [11] |
| Endurance capacity | ↑ ↑ ↑ | ↔ ↑ | [11] |
| Capillary growth (angiogenesis) | ↑ ↑ | ↔ | [11] |
| Mitochondrial biogenesis | ↑ ↑ | ↔ ↑ | [11] |
| Mitochondrial density and oxidative function | ↑ ↑ ↑ | ↔ ↑ | [11] |
| Bone mineral density | ↑ ↑ | ↑ ↑ | [11] |
| Inflammatory markers | ↓ ↓ | ↓ | [11] |
| Flexibility | ↑ | ↑ | [11] |
| Posture | ↔ | ↑ | [11] |
| Ability in activities of daily living | ↔ ↑ | ↑ ↑ | [11] |
| Basal metabolic rate | ↑ | ↑ ↑ | [11] |
| Percent body fat | ↓ ↓ | ↓ | [11] |
| Lean body mass | ↔ | ↑ ↑ | [11] |
| Resting insulin levels | ↓ | ↓ | [11] |
| Insulin sensitivity | ↑ ↑ | ↑ ↑ | [11] |
| Insulin response to glucose challenge | ↓ ↓ | ↓ ↓ | [11] |
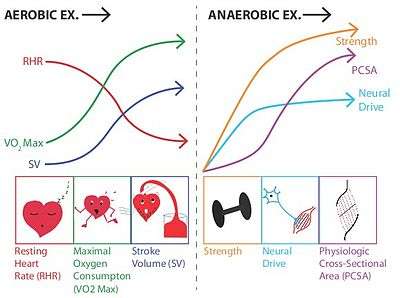
| Resting heart rate | ↓ ↓ | ↔ | [11] |
| Stroke volume (resting and maximal) | ↑ ↑ | ↔ | [11] |
| Systolic blood pressure (resting) | ↔ ↓ | ↔ | [11] |
| Diastolic blood pressure (resting) | ↔ ↓ | ↔ ↓ | [11] |
| Cardiovascular risk profile | ↓ ↓ ↓ | ↓ | [11] |
Table legend
| |||
Physical exercise is important for maintaining physical fitness and can contribute to maintaining a healthy weight, regulating the digestive system, building and maintaining healthy bone density, muscle strength, and joint mobility, promoting physiological well-being, reducing surgical risks, and strengthening the immune system. Some studies indicate that exercise may increase life expectancy and the overall quality of life.[12] People who participate in moderate to high levels of physical exercise have a lower mortality rate compared to individuals who by comparison are not physically active.[13] Moderate levels of exercise have been correlated with preventing aging by reducing inflammatory potential.[14] The majority of the benefits from exercise are achieved with around 3500 metabolic equivalent (MET) minutes per week, with diminishing returns at higher levels of activity.[15] For example, climbing stairs 10 minutes, vacuuming 15 minutes, gardening 20 minutes, running 20 minutes, and walking or bicycling for transportation 25 minutes on a daily basis would together achieve about 3000 MET minutes a week.[15] A lack of physical activity causes approximately 6% of the burden of disease from coronary heart disease, 7% of type 2 diabetes, 10% of breast cancer and 10% of colon cancer worldwide.[16] Overall, physical inactivity causes 9% of premature mortality worldwide.[16]
Fitness
Individuals can increase fitness by increasing physical activity levels.[17] Increases in muscle size from resistance training are primarily determined by diet and testosterone.[18] This genetic variation in improvement from training is one of the key physiological differences between elite athletes and the larger population.[19][20] Studies have shown that exercising in middle age leads to better physical ability later in life.[21]
Early motor skills and development is also related to physical activity and performance later in life. Children who are more proficient with motor skills early on are more inclined to be physically active, and thus tend to perform well in sports and have better fitness levels. Early motor proficiency has a positive correlation to childhood physical activity and fitness levels, while less proficiency in motor skills results in a more sedentary lifestyle.[22]
A 2015 meta-analysis demonstrated that high-intensity interval training improved one's VO2 max more than lower intensity endurance training.[23]
Cardiovascular system
The beneficial effect of exercise on the cardiovascular system is well documented. There is a direct correlation between physical inactivity and cardiovascular mortality, and physical inactivity is an independent risk factor for the development of coronary artery disease. Low levels of physical exercise increase the risk of cardiovascular diseases mortality.[24]
Children who participate in physical exercise experience greater loss of body fat and increased cardiovascular fitness.[25] Studies have shown that academic stress in youth increases the risk of cardiovascular disease in later years; however, these risks can be greatly decreased with regular physical exercise.[26] There is a dose-response relationship between the amount of exercise performed from approximately 700–2000 kcal of energy expenditure per week and all-cause mortality and cardiovascular disease mortality in middle-aged and elderly men. The greatest potential for reduced mortality is seen in sedentary individuals who become moderately active. Studies have shown that since heart disease is the leading cause of death in women, regular exercise in aging women leads to healthier cardiovascular profiles. Most beneficial effects of physical activity on cardiovascular disease mortality can be attained through moderate-intensity activity (40–60% of maximal oxygen uptake, depending on age). Persons who modify their behavior after myocardial infarction to include regular exercise have improved rates of survival. Persons who remain sedentary have the highest risk for all-cause and cardiovascular disease mortality.[27] According to the American Heart Association, exercise reduces the risk of cardiovascular diseases, including heart attack and stroke.[24]
Immune system
Although there have been hundreds of studies on physical exercise and the immune system, there is little direct evidence on its connection to illness.[28] Epidemiological evidence suggests that moderate exercise has a beneficial effect on the human immune system; an effect which is modeled in a J curve. Moderate exercise has been associated with a 29% decreased incidence of upper respiratory tract infections (URTI), but studies of marathon runners found that their prolonged high-intensity exercise was associated with an increased risk of infection occurrence.[28] However, another study did not find the effect. Immune cell functions are impaired following acute sessions of prolonged, high-intensity exercise, and some studies have found that athletes are at a higher risk for infections. Studies have shown that strenuous stress for long durations, such as training for a marathon, can suppress the immune system by decreasing the concentration of lymphocytes.[29] The immune systems of athletes and nonathletes are generally similar. Athletes may have a slightly elevated natural killer cell count and cytolytic action, but these are unlikely to be clinically significant.[28]
Vitamin C supplementation has been associated with a lower incidence of upper respiratory tract infections in marathon runners.[28]
Biomarkers of inflammation such as C-reactive protein, which are associated with chronic diseases, are reduced in active individuals relative to sedentary individuals, and the positive effects of exercise may be due to its anti-inflammatory effects. In individuals with heart disease, exercise interventions lower blood levels of fibrinogen and C-reactive protein, an important cardiovascular risk marker.[30] The depression in the immune system following acute bouts of exercise may be one of the mechanisms for this anti-inflammatory effect.[28]
Cancer
A systematic review evaluated 45 studies that examined the relationship between physical activity and cancer survival rates. According to the review, "[there] was consistent evidence from 27 observational studies that physical activity is associated with reduced all-cause, breast cancer–specific, and colon cancer–specific mortality. There is currently insufficient evidence regarding the association between physical activity and mortality for survivors of other cancers."[31] Evidence suggests that exercise may positively affect cancer survivors health-related quality of life, including factors such as anxiety, self-esteem and emotional well-being.[32] For people with cancer undergoing active treatment, exercise may also have positive effects on health-related quality of life, such as fatigue and physical functioning.[33] This is likely to be more pronounced with higher intensity exercise.[33] Although there is only limited scientific evidence on the subject, people with cancer cachexia are encouraged to engage in physical exercise.[34] Due to various factors, some individuals with cancer cachexia have a limited capacity for physical exercise.[35][36] Compliance with prescribed exercise is low in individuals with cachexia and clinical trials of exercise in this population often suffer from high drop-out rates.[35][36]
The evidence is very uncertain about the effect of aerobic physical exercises on anxiety and serious adverse events for adults with haematological malignancies.[37] Aerobic physical exercises may result in little to no difference in the mortality, in the quality of life and in the physical functioning.[37] These exercises may result in a slight reduction in depression. Furthermore, aerobic physical exercises probably reduce fatigue.[37]
Neurobiological
The neurobiological effects of physical exercise are numerous and involve a wide range of interrelated effects on brain structure, brain function, and cognition.[38][39][40][41] A large body of research in humans has demonstrated that consistent aerobic exercise (e.g., 30 minutes every day) induces persistent improvements in certain cognitive functions, healthy alterations in gene expression in the brain, and beneficial forms of neuroplasticity and behavioral plasticity; some of these long-term effects include: increased neuron growth, increased neurological activity (e.g., c-Fos and BDNF signaling), improved stress coping, enhanced cognitive control of behavior, improved declarative, spatial, and working memory, and structural and functional improvements in brain structures and pathways associated with cognitive control and memory.[38][39][40][41][42][43][44][45][46][47] The effects of exercise on cognition have important implications for improving academic performance in children and college students, improving adult productivity, preserving cognitive function in old age, preventing or treating certain neurological disorders, and improving overall quality of life.[38][48][49]
In healthy adults, aerobic exercise has been shown to induce transient effects on cognition after a single exercise session and persistent effects on cognition following regular exercise over the course of several months.[38][47][50] People who regularly perform aerobic exercise (e.g., running, jogging, brisk walking, swimming, and cycling) have greater scores on neuropsychological function and performance tests that measure certain cognitive functions, such as attentional control, inhibitory control, cognitive flexibility, working memory updating and capacity, declarative memory, spatial memory, and information processing speed.[38][42][44][46][47][50] The transient effects of exercise on cognition include improvements in most executive functions (e.g., attention, working memory, cognitive flexibility, inhibitory control, problem solving, and decision making) and information processing speed for a period of up to 2 hours after exercising.[50]
Aerobic exercise induces short- and long-term effects on mood and emotional states by promoting positive affect, inhibiting negative affect, and decreasing the biological response to acute psychological stress.[50] Over the short-term, aerobic exercise functions as both an antidepressant and euphoriant,[51][52][53][54] whereas consistent exercise produces general improvements in mood and self-esteem.[55][56]
Regular aerobic exercise improves symptoms associated with a variety of central nervous system disorders and may be used as an adjunct therapy for these disorders. There is clear evidence of exercise treatment efficacy for major depressive disorder and attention deficit hyperactivity disorder.[48][53][57][58][59][60] The American Academy of Neurology's clinical practice guideline for mild cognitive impairment indicates that clinicians should recommend regular exercise (two times per week) to individuals who have been diagnosed with this condition.[61] Reviews of clinical evidence also support the use of exercise as an adjunct therapy for certain neurodegenerative disorders, particularly Alzheimer’s disease and Parkinson's disease.[62][63][64][65][66][67] Regular exercise is also associated with a lower risk of developing neurodegenerative disorders.[65][68] A large body of preclinical evidence and emerging clinical evidence supports the use of exercise as an adjunct therapy for the treatment and prevention of drug addictions.[69][70][71][72][73] Regular exercise has also been proposed as an adjunct therapy for brain cancers.[74]Depression
A number of medical reviews have indicated that exercise has a marked and persistent antidepressant effect in humans,[42][53][54][57][75][76] an effect believed to be mediated through enhanced BDNF signaling in the brain.[45][57] Several systematic reviews have analyzed the potential for physical exercise in the treatment of depressive disorders. The 2013 Cochrane Collaboration review on physical exercise for depression noted that, based upon limited evidence, it is more effective than a control intervention and comparable to psychological or antidepressant drug therapies.[75] Three subsequent 2014 systematic reviews that included the Cochrane review in their analysis concluded with similar findings: one indicated that physical exercise is effective as an adjunct treatment (i.e., treatments that are used together) with antidepressant medication;[57] the other two indicated that physical exercise has marked antidepressant effects and recommended the inclusion of physical activity as an adjunct treatment for mild–moderate depression and mental illness in general.[53][54] One systematic review noted that yoga may be effective in alleviating symptoms of prenatal depression.[77] Another review asserted that evidence from clinical trials supports the efficacy of physical exercise as a treatment for depression over a 2–4 month period.[42] These benefits have also been noted in old age, with a review conducted in 2019 finding that exercise is an effective treatment for clinically diagnosed depression in older adults.[78]
A 2015 review of clinical evidence which included a medical guideline for the treatment of depression with exercise noted that the available evidence on the effectiveness of exercise therapy for depression suffers from some limitations;[58] nonetheless, it stated that there is clear evidence of efficacy for reducing symptoms of depression.[58] The review also noted that patient characteristics, the type of depressive disorder, and the nature of the exercise program all affect the antidepressant properties of exercise therapy.[58] A meta-analysis from July 2016 concluded that physical exercise improves overall quality of life in individuals with depression relative to controls.[48]Continuous aerobic exercise can induce a transient state of euphoria, colloquially known as a "runner's high" in distance running or a "rower's high" in crew, through the increased biosynthesis of at least three euphoriant neurochemicals: anandamide (an endocannabinoid),[79] β-endorphin (an endogenous opioid),[80] and phenethylamine (a trace amine and amphetamine analog).[81][82][83]
Sleep
Preliminary evidence from a 2012 review indicated that physical training for up to four months may increase sleep quality in adults over 40 years of age.[84] A 2010 review suggested that exercise generally improved sleep for most people, and may help with insomnia, but there is insufficient evidence to draw detailed conclusions about the relationship between exercise and sleep.[85] A 2018 systematic review and meta-analysis suggested that exercise can improve sleep quality in people with insomnia.[86]
Libido
One 2013 study found that exercising improved sexual arousal problems related to antidepressant use.[87]
Mechanism of effects
Skeletal muscle
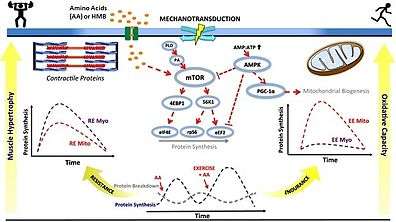
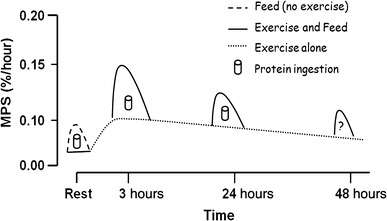
Resistance training and subsequent consumption of a protein-rich meal promotes muscle hypertrophy and gains in muscle strength by stimulating myofibrillar muscle protein synthesis (MPS) and inhibiting muscle protein breakdown (MPB).[88][89] The stimulation of muscle protein synthesis by resistance training occurs via phosphorylation of the mechanistic target of rapamycin (mTOR) and subsequent activation of mTORC1, which leads to protein biosynthesis in cellular ribosomes via phosphorylation of mTORC1's immediate targets (the p70S6 kinase and the translation repressor protein 4EBP1).[88][90] The suppression of muscle protein breakdown following food consumption occurs primarily via increases in plasma insulin.[88][91][92] Similarly, increased muscle protein synthesis (via activation of mTORC1) and suppressed muscle protein breakdown (via insulin-independent mechanisms) has also been shown to occur following ingestion of β-hydroxy β-methylbutyric acid.[88][91][92][93]
Aerobic exercise induces mitochondrial biogenesis and an increased capacity for oxidative phosphorylation in the mitochondria of skeletal muscle, which is one mechanism by which aerobic exercise enhances submaximal endurance performance.[94] [88][95] These effects occur via an exercise-induced increase in the intracellular AMP:ATP ratio, thereby triggering the activation of AMP-activated protein kinase (AMPK) which subsequently phosphorylates peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α), the master regulator of mitochondrial biogenesis.[88][95][96]
• PA: phosphatidic acid
• mTOR: mechanistic target of rapamycin
• AMP: adenosine monophosphate
• ATP: adenosine triphosphate
• AMPK: AMP-activated protein kinase
• PGC‐1α: peroxisome proliferator-activated receptor gamma coactivator-1α
• S6K1: p70S6 kinase
• 4EBP1: eukaryotic translation initiation factor 4E-binding protein 1
• eIF4E: eukaryotic translation initiation factor 4E
• RPS6: ribosomal protein S6
• eEF2: eukaryotic elongation factor 2
• RE: resistance exercise; EE: endurance exercise
• Myo: myofibrillar; Mito: mitochondrial
• AA: amino acids
• HMB: β-hydroxy β-methylbutyric acid
• ↑ represents activation
• Τ represents inhibition
Other peripheral organs
Developing research has demonstrated that many of the benefits of exercise are mediated through the role of skeletal muscle as an endocrine organ. That is, contracting muscles release multiple substances known as myokines which promote the growth of new tissue, tissue repair, and multiple anti-inflammatory functions, which in turn reduce the risk of developing various inflammatory diseases.[110] Exercise reduces levels of cortisol, which causes many health problems, both physical and mental.[111] Endurance exercise before meals lowers blood glucose more than the same exercise after meals.[112] There is evidence that vigorous exercise (90–95% of VO2 max) induces a greater degree of physiological cardiac hypertrophy than moderate exercise (40 to 70% of VO2 max), but it is unknown whether this has any effects on overall morbidity and/or mortality.[113] Both aerobic and anaerobic exercise work to increase the mechanical efficiency of the heart by increasing cardiac volume (aerobic exercise), or myocardial thickness (strength training). Ventricular hypertrophy, the thickening of the ventricular walls, is generally beneficial and healthy if it occurs in response to exercise.
Public health measures
Multiple component community-wide campaigns are frequently used in an attempt to increase a population's level of physical activity. A 2015 Cochrane review, however, did not find evidence supporting a benefit.[120] The quality of the underlying evidence was also poor.[120] However, there is some evidence that school-based interventions can increase activity levels and fitness in children.[17] Another Cochrane review found some evidence that certain types of exercise programmes, such as those involving gait, balance, co-ordination and functional tasks, can improve balance in older adults.[121] Following progressive resistance training, older adults also respond with improved physical function.[122] Survey of brief interventions promoting physical activity found that they are cost-effective, although there are variations between studies.[123]
Environmental approaches appear promising: signs that encourage the use of stairs, as well as community campaigns, may increase exercise levels.[124] The city of Bogotá, Colombia, for example, blocks off 113 kilometers (70 mi) of roads on Sundays and holidays to make it easier for its citizens to get exercise. Such pedestrian zones are part of an effort to combat chronic diseases and to maintain a healthy BMI.[125][126]
To identify which public health strategies are effective, a Cochrane overview of reviews is in preparation.[127]
Physical exercise was said to decrease healthcare costs, increase the rate of job attendance, as well as increase the amount of effort women put into their jobs.[128] There is some level of concern about additional exposure to air pollution when exercising outdoors, especially near traffic.[129]
Children will mimic the behavior of their parents in relation to physical exercise. Parents can thus promote physical activity and limit the amount of time children spend in front of screens.[130]
Children who are overweight and participate in physical exercise experience a greater loss of body fat and increased cardiovascular fitness. According to the Centers for Disease Control and Prevention in the United States, children and adolescents should do 60 minutes or more of physical activity each day.[131] Implementing physical exercise in the school system and ensuring an environment in which children can reduce barriers to maintain a healthy lifestyle is essential.
The European Commission's Directorate-General for Education and Culture (DG EAC) has dedicated programs and funds for Health Enhancing Physical Activity (HEPA) projects[132] within its Horizon 2020 and Erasmus+ program, as research showed that too many Europeans are not physically active enough. Financing is available for increased collaboration between players active in this field across the EU and around the world, the promotion of HEPA in the EU and its partner countries, and the European Sports Week. The DG EAC regularly publishes a Eurobarometer on sport and physical activity.
Exercise trends

Worldwide there has been a large shift toward less physically demanding work.[133] This has been accompanied by increasing use of mechanized transportation, a greater prevalence of labor-saving technology in the home, and fewer active recreational pursuits.[133] Personal lifestyle changes, however, can correct the lack of physical exercise.
Research published in 2015 suggests that incorporating mindfulness into physical exercise interventions increases exercise adherence and self-efficacy, and also has positive effects both psychologically and physiologically.[134]
Social and cultural variation
Exercising looks different in every country, as do the motivations behind exercising.[3] In some countries, people exercise primarily indoors, while in others, people primarily exercise outdoors. People may exercise for personal enjoyment, health and well-being, social interactions, competition or training, etc. These differences could potentially be attributed to a variety of reasons including geographic location and social tendencies.
In Colombia, for example, citizens value and celebrate the outdoor environments of their country. In many instances, they utilize outdoor activities as social gatherings to enjoy nature and their communities. In Bogotá, Colombia, a 70-mile stretch of road known as the Ciclovía is shut down each Sunday for bicyclists, runners, rollerbladers, skateboarders and other exercisers to work out and enjoy their surroundings.[135]
Similarly to Colombia, citizens of Cambodia tend to exercise socially outside. In this country, public gyms have become quite popular. People will congregate at these outdoor gyms not only to utilize the public facilities, but also to organize aerobics and dance sessions, which are open to the public.[136]
Sweden has also begun developing outdoor gyms, called utegym. These gyms are free to the public and are often placed in beautiful, picturesque environments. People will swim in rivers, use boats, and run through forests to stay healthy and enjoy the natural world around them. This works particularly well in Sweden due to its geographical location.[137]
Exercise in some areas of China, particularly among those who are retired, seems to be socially grounded. In the mornings, dances are held in public parks; these gatherings may include Latin dancing, ballroom dancing, tango, or even the jitterbug. Dancing in public allows people to interact with those with whom they would not normally interact, allowing for both health and social benefits.[138]
These sociocultural variations in physical exercise show how people in different geographic locations and social climates have varying motivations and methods of exercising. Physical exercise can improve health and well-being, as well as enhance community ties and appreciation of natural beauty.[3]
Nutrition and recovery
Proper nutrition is as important to health as exercise. When exercising, it becomes even more important to have a good diet to ensure that the body has the correct ratio of macronutrients while providing ample micronutrients, in order to aid the body with the recovery process following strenuous exercise.[139]
Active recovery is recommended after participating in physical exercise because it removes lactate from the blood more quickly than inactive recovery. Removing lactate from circulation allows for an easy decline in body temperature, which can also benefit the immune system, as an individual may be vulnerable to minor illnesses if the body temperature drops too abruptly after physical exercise.[140]
Excessive exercise
Excessive exercise or overtraining occurs when a person exceeds their body's ability to recover from strenuous exercise.[141]
History

The benefits of exercise have been known since antiquity. Dating back to 65 BCE, it was Marcus Cicero, Roman politician and lawyer, who stated: "It is exercise alone that supports the spirits, and keeps the mind in vigor."[142] Exercise was also seen to be valued later in history during the Early Middle Ages as a means of survival by the Germanic peoples of Northern Europe.[143]
More recently, exercise was regarded as a beneficial force in the 19th century. After 1860, Archibald MacLaren opened a gymnasium at the University of Oxford and instituted a training regimen for 12 military officials at the university.[144] This regimen was assimilated into the training of the British Army, which formed the Army Gymnastic Staff in 1860 and made sport an important part of military life.[145][146][147] Several mass exercise movements were started in the early twentieth century as well. The first and most significant of these in the UK was the Women's League of Health and Beauty, founded in 1930 by Mary Bagot Stack, that had 166,000 members in 1937.[148]
The link between physical health and exercise (or lack of it) was further established in 1949 and reported in 1953 by a team led by Jerry Morris.[149][150] Dr. Morris noted that men of similar social class and occupation (bus conductors versus bus drivers) had markedly different rates of heart attacks, depending on the level of exercise they got: bus drivers had a sedentary occupation and a higher incidence of heart disease, while bus conductors were forced to move continually and had a lower incidence of heart disease.[150]
Other animals
Studies of animals indicate that physical activity may be more adaptable than changes in food intake to regulate energy balance.[151]
Mice having access to activity wheels engaged in voluntary exercise and increased their propensity to run as adults.[152] Artificial selection of mice exhibited significant heritability in voluntary exercise levels,[153] with "high-runner" breeds having enhanced aerobic capacity,[154] hippocampal neurogenesis,[155] and skeletal muscle morphology.[156]
The effects of exercise training appear to be heterogeneous across non-mammalian species. As examples, exercise training of salmon showed minor improvements of endurance,[157] and a forced swimming regimen of yellowtail amberjack and rainbow trout accelerated their growth rates and altered muscle morphology favorable for sustained swimming.[158][159] Crocodiles, alligators, and ducks showed elevated aerobic capacity following exercise training.[160][161][162] No effect of endurance training was found in most studies of lizards,[160][163] although one study did report a training effect.[164] In lizards, sprint training had no effect on maximal exercise capacity,[164] and muscular damage from over-training occurred following weeks of forced treadmill exercise.[163]
See also
- Active living
- Behavioural change theories
- Bodybuilding
- Exercise hypertension
- Exercise-induced nausea
- Exercise intensity
- Exercise intolerance
- Exercise-induced anaphylaxis
- Exercise-induced asthma
- Kinesiology
- Metabolic equivalent
- Non-exercise associated thermogenesis
- Neurobiological effects of physical exercise
- Supercompensation
- Warming up
References
- Kylasov A, Gavrov S (2011). Diversity Of Sport: non-destructive evaluation. Paris: UNESCO: Encyclopedia of Life Support Systems. pp. 462–91. ISBN 978-5-89317-227-0.
- "7 great reasons why exercise matters". Mayo Clinic. Retrieved 2 November 2018.
- Bergstrom, Kristine; Muse, Toby; Tsai, Michelle; Strangio, Sebastian. "Fitness for Foreigners". Slate Magazine. Slate Magazine. Retrieved 5 December 2016.
- "Exercise". UK NHS Live Well. 26 April 2018. Retrieved 13 November 2019.
- National Institutes of Health, National Heart, Lung, and Blood Institute (June 2006). "Your Guide to Physical Activity and Your Heart" (PDF). U.S. Department of Health and Human Services.
- Wilmore J.; Knuttgen H. (2003). "Aerobic Exercise and Endurance Improving Fitness for Health Benefits". The Physician and Sportsmedicine. 31 (5): 45–51. doi:10.3810/psm.2003.05.367. PMID 20086470.
- De Vos N.; Singh N.; Ross D.; Stavrinos T. (2005). "Optimal Load for Increasing Muscle Power During Explosive Resistance Training in Older Adults". The Journals of Gerontology. 60A (5): 638–47. doi:10.1093/gerona/60.5.638. PMID 15972618.
- O'Connor D.; Crowe M.; Spinks W. (2005). "Effects of static stretching on leg capacity during cycling". Turin. 46 (1): 52–56.
- "What Is Fitness?" (PDF). The CrossFit Journal. October 2002. p. 4. Retrieved 12 September 2010.
- de Souza Nery S, Gomides RS, da Silva GV, de Moraes Forjaz CL, Mion D Jr, Tinucci T (1 March 2010). "Intra-Arterial Blood Pressure Response in Hypertensive Subjects during Low- and High-Intensity Resistance Exercise". Clinics. 65 (3): 271–77. doi:10.1590/S1807-59322010000300006. PMC 2845767. PMID 20360917.
- Egan B, Zierath JR (February 2013). "Exercise metabolism and the molecular regulation of skeletal muscle adaptation". Cell Metabolism. 17 (2): 162–84. doi:10.1016/j.cmet.2012.12.012. PMID 23395166.
- Gremeaux, V; Gayda, M; Lepers, R; Sosner, P; Juneau, M; Nigam, A (December 2012). "Exercise and longevity". Maturitas. 73 (4): 312–17. doi:10.1016/j.maturitas.2012.09.012. PMID 23063021.
- Department Of Health And Human Services, United States (1996). "Physical Activity and Health". United States Department of Health. ISBN 978-1-4289-2794-0.
- Woods, Jeffrey A.; Wilund, Kenneth R.; Martin, Stephen A.; Kistler, Brandon M. (29 October 2011). "Exercise, Inflammation and Aging". Aging and Disease. 3 (1): 130–40. PMC 3320801. PMID 22500274.
- Kyu, Hmwe H; Bachman, Victoria F; Alexander, Lily T; Mumford, John Everett; Afshin, Ashkan; Estep, Kara; Veerman, J Lennert; Delwiche, Kristen; Iannarone, Marissa L; Moyer, Madeline L; Cercy, Kelly; Vos, Theo; Murray, Christopher J L; Forouzanfar, Mohammad H (9 August 2016). "Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013". BMJ. 354: i3857. doi:10.1136/bmj.i3857. PMC 4979358. PMID 27510511.
- Lee, I-Min; Shiroma, Eric J; Lobelo, Felipe; Puska, Pekka; Blair, Steven N; Katzmarzyk, Peter T (21 July 2012). "Impact of Physical Inactivity on the World's Major Non-Communicable Diseases". Lancet. 380 (9838): 219–29. doi:10.1016/S0140-6736(12)61031-9. PMC 3645500. PMID 22818936.
- Dobbins, Maureen; Husson, Heather; DeCorby, Kara; LaRocca, Rebecca L (28 February 2013). Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd. pp. CD007651. doi:10.1002/14651858.cd007651.pub2. PMC 7197501. PMID 23450577. S2CID 205190823.
- Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Seip RL, Clarkson PM (June 2005). "Variability in muscle size and strength gain after unilateral resistance training". Medicine & Science in Sports & Exercise. 37 (6): 964–72. PMID 15947721.
- Brutsaert TD, Parra EJ (2006). "What makes a champion? Explaining variation in human athletic performance". Respiratory Physiology & Neurobiology. 151 (2–3): 109–23. doi:10.1016/j.resp.2005.12.013. PMID 16448865.
- Geddes, Linda (28 July 2007). "Superhuman". New Scientist. pp. 35–41.
- "Being active combats risk of functional problems".
- Wrotniak, B.H; Epstein, L.H; Dorn, J.M; Jones, K.E; Kondilis, V.A (2006). "The Relationship Between Motor Proficiency and Physical Activity in Children". Pediatrics. 118 (6): e1758-65. doi:10.1542/peds.2006-0742. PMID 17142498.
- Milanović, Zoran; Sporiš, Goran; Weston, Matthew (2015). "Effectiveness of High-Intensity Interval Training (HIIT) and Continuous Endurance Training for VO2max Improvements: A Systematic Review and Meta-Analysis of Controlled Trials" (PDF). Sports Medicine. 45 (10): 1469–81. doi:10.1007/s40279-015-0365-0. PMID 26243014.
- "American Heart Association Recommendations for Physical Activity in Adults". American Heart Association. 14 December 2017. Retrieved 5 May 2018.
- Lumeng, Julie C (2006). "Small-group physical education classes result in important health benefits". The Journal of Pediatrics. 148 (3): 418–19. doi:10.1016/j.jpeds.2006.02.025. PMID 17243298.
- Ahaneku, Joseph E.; Nwosu, Cosmas M.; Ahaneku, Gladys I. (2000). "Academic Stress and Cardiovascular Health". Academic Medicine. 75 (6): 567–68. doi:10.1097/00001888-200006000-00002. PMID 10875499.
- Fletcher, G.F; Balady, G; Blair, S.N.; Blumenthal, J; Caspersen, C; Chaitman, B; Epstein, S; Froelicher, E.S.S; Froelicher, V.F.; Pina, I.L; Pollock, M.L (1996). "Statement on Exercise: Benefits and Recommendations for Physical Activity Programs for All Americans: A Statement for Health Professionals by the Committee on Exercise and Cardiac Rehabilitation of the Council on Clinical Cardiology, American Heart Association". Circulation. 94 (4): 857–62. doi:10.1161/01.CIR.94.4.857. PMID 8772712.
- Gleeson M (August 2007). "Immune function in sport and exercise". J. Appl. Physiol. 103 (2): 693–99. doi:10.1152/japplphysiol.00008.2007. PMID 17303714. S2CID 18112931.
- Goodman, C. C.; Kapasi, Z.F. (2002). "The effect of exercise on the immune system". Rehabilitation Oncology. 20: 13–15. doi:10.1097/01893697-200220010-00013.
- Swardfager W (2012). "Exercise intervention and inflammatory markers in coronary artery disease: a meta-analysis". Am. Heart J. 163 (4): 666–76. doi:10.1016/j.ahj.2011.12.017. PMID 22520533.
- Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM (2012). "Physical Activity, Biomarkers, and Disease Outcomes in Cancer Survivors: A Systematic Review". JNCI Journal of the National Cancer Institute. 104 (11): 815–40. doi:10.1093/jnci/djs207. PMC 3465697. PMID 22570317.
- Mishra, Shiraz I; Scherer, Roberta W; Geigle, Paula M; Berlanstein, Debra R; Topaloglu, Ozlem; Gotay, Carolyn C; Snyder, Claire (15 August 2012). "Exercise interventions on health-related quality of life for cancer survivors". Cochrane Database of Systematic Reviews (8): CD007566. doi:10.1002/14651858.cd007566.pub2. ISSN 1465-1858. PMID 22895961.
- Mishra, Shiraz I; Scherer, Roberta W; Snyder, Claire; Geigle, Paula M; Berlanstein, Debra R; Topaloglu, Ozlem (15 August 2012). "Exercise interventions on health-related quality of life for people with cancer during active treatment". Cochrane Database of Systematic Reviews (8): CD008465. doi:10.1002/14651858.cd008465.pub2. ISSN 1465-1858. PMID 22895974.
- Grande AJ, Silva V, Maddocks M (September 2015). "Exercise for cancer cachexia in adults: Executive summary of a Cochrane Collaboration systematic review". Journal of Cachexia, Sarcopenia and Muscle. 6 (3): 208–11. doi:10.1002/jcsm.12055. PMC 4575551. PMID 26401466.
- Sadeghi M, Keshavarz-Fathi M, Baracos V, Arends J, Mahmoudi M, Rezaei N (July 2018). "Cancer cachexia: Diagnosis, assessment, and treatment". Crit. Rev. Oncol. Hematol. 127: 91–104. doi:10.1016/j.critrevonc.2018.05.006. PMID 29891116.
- Solheim TS, Laird BJ, Balstad TR, Bye A, Stene G, Baracos V, Strasser F, Griffiths G, Maddocks M, Fallon M, Kaasa S, Fearon K (February 2018). "Cancer cachexia: rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial". BMJ Support Palliat Care. 8 (3): 258–265. doi:10.1136/bmjspcare-2017-001440. PMID 29440149.
- Knips, Linus; Bergenthal, Nils; Streckmann, Fiona; Monsef, Ina; Elter, Thomas; Skoetz, Nicole (31 January 2019). Cochrane Haematological Malignancies Group (ed.). "Aerobic physical exercise for adult patients with haematological malignancies". Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD009075.pub3.
- Erickson KI, Hillman CH, Kramer AF (August 2015). "Physical activity, brain, and cognition". Current Opinion in Behavioral Sciences. 4: 27–32. doi:10.1016/j.cobeha.2015.01.005.
- Paillard T, Rolland Y, de Souto Barreto P (July 2015). "Protective Effects of Physical Exercise in Alzheimer's Disease and Parkinson's Disease: A Narrative Review". J Clin Neurol. 11 (3): 212–219. doi:10.3988/jcn.2015.11.3.212. PMC 4507374. PMID 26174783.
Aerobic physical exercise (PE) activates the release of neurotrophic factors and promotes angiogenesis, thereby facilitating neurogenesis and synaptogenesis, which in turn improve memory and cognitive functions. ... Exercise limits the alteration in dopaminergic neurons in the substantia nigra and contributes to optimal functioning of the basal ganglia involved in motor commands and control by adaptive mechanisms involving dopamine and glutamate neurotransmission.
- McKee AC, Daneshvar DH, Alvarez VE, Stein TD (January 2014). "The neuropathology of sport". Acta Neuropathol. 127 (1): 29–51. doi:10.1007/s00401-013-1230-6. PMC 4255282. PMID 24366527.
The benefits of regular exercise, physical fitness and sports participation on cardiovascular and brain health are undeniable ... Exercise also enhances psychological health, reduces age-related loss of brain volume, improves cognition, reduces the risk of developing dementia, and impedes neurodegeneration.
- Denham J, Marques FZ, O'Brien BJ, Charchar FJ (February 2014). "Exercise: putting action into our epigenome". Sports Med. 44 (2): 189–209. doi:10.1007/s40279-013-0114-1. PMID 24163284.
Aerobic physical exercise produces numerous health benefits in the brain. Regular engagement in physical exercise enhances cognitive functioning, increases brain neurotrophic proteins, such as brain-derived neurotrophic factor (BDNF), and prevents cognitive diseases [76–78]. Recent findings highlight a role for aerobic exercise in modulating chromatin remodelers [21, 79–82]. ... These results were the first to demonstrate that acute and relatively short aerobic exercise modulates epigenetic modifications. The transient epigenetic modifications observed due to chronic running training have also been associated with improved learning and stress-coping strategies, epigenetic changes and increased c-Fos-positive neurons ... Nonetheless, these studies demonstrate the existence of epigenetic changes after acute and chronic exercise and show they are associated with improved cognitive function and elevated markers of neurotrophic factors and neuronal activity (BDNF and c-Fos). ... The aerobic exercise training-induced changes to miRNA profile in the brain seem to be intensity-dependent [164]. These few studies provide a basis for further exploration into potential miRNAs involved in brain and neuronal development and recovery via aerobic exercise.
- Gomez-Pinilla F, Hillman C (January 2013). The influence of exercise on cognitive abilities. Compr. Physiol. 3. pp. 403–428. doi:10.1002/cphy.c110063. ISBN 9780470650714. PMC 3951958. PMID 23720292.
- Erickson KI, Leckie RL, Weinstein AM (September 2014). "Physical activity, fitness, and gray matter volume". Neurobiol. Aging. 35 Suppl 2: S20–528. doi:10.1016/j.neurobiolaging.2014.03.034. PMC 4094356. PMID 24952993.
- Guiney H, Machado L (February 2013). "Benefits of regular aerobic exercise for executive functioning in healthy populations". Psychon Bull Rev. 20 (1): 73–86. doi:10.3758/s13423-012-0345-4. PMID 23229442.
- Erickson KI, Miller DL, Roecklein KA (2012). "The aging hippocampus: interactions between exercise, depression, and BDNF". Neuroscientist. 18 (1): 82–97. doi:10.1177/1073858410397054. PMC 3575139. PMID 21531985.
- Buckley J, Cohen JD, Kramer AF, McAuley E, Mullen SP (2014). "Cognitive control in the self-regulation of physical activity and sedentary behavior". Front Hum Neurosci. 8: 747. doi:10.3389/fnhum.2014.00747. PMC 4179677. PMID 25324754.
- Cox EP, O'Dwyer N, Cook R, Vetter M, Cheng HL, Rooney K, O'Connor H (August 2016). "Relationship between physical activity and cognitive function in apparently healthy young to middle-aged adults: A systematic review". J. Sci. Med. Sport. 19 (8): 616–628. doi:10.1016/j.jsams.2015.09.003. PMID 26552574.
A range of validated platforms assessed CF across three domains: executive function (12 studies), memory (four studies) and processing speed (seven studies). ... In studies of executive function, five found a significant ES in favour of higher PA, ranging from small to large. Although three of four studies in the memory domain reported a significant benefit of higher PA, there was only one significant ES, which favoured low PA. Only one study examining processing speed had a significant ES, favouring higher PA.
CONCLUSIONS: A limited body of evidence supports a positive effect of PA on CF in young to middle-aged adults. Further research into this relationship at this age stage is warranted. ...
Significant positive effects of PA on cognitive function were found in 12 of the 14 included manuscripts, the relationship being most consistent for executive function, intermediate for memory and weak for processing speed. - Schuch FB, Vancampfort D, Rosenbaum S, Richards J, Ward PB, Stubbs B (July 2016). "Exercise improves physical and psychological quality of life in people with depression: A meta-analysis including the evaluation of control group response". Psychiatry Res. 241: 47–54. doi:10.1016/j.psychres.2016.04.054. PMID 27155287.
Exercise has established efficacy as an antidepressant in people with depression. ... Exercise significantly improved physical and psychological domains and overall QoL. ... The lack of improvement among control groups reinforces the role of exercise as a treatment for depression with benefits to QoL.
- Pratali L, Mastorci F, Vitiello N, Sironi A, Gastaldelli A, Gemignani A (November 2014). "Motor Activity in Aging: An Integrated Approach for Better Quality of Life". International Scholarly Research Notices. 2014: 257248. doi:10.1155/2014/257248. PMC 4897547. PMID 27351018.
Research investigating the effects of exercise on older adults has primarily focused on brain structural and functional changes with relation to cognitive improvement. In particular, several cross-sectional and intervention studies have shown a positive association between physical activity and cognition in older persons [86] and an inverse correlation with cognitive decline and dementia [87]. Older adults enrolled in a 6-month aerobic fitness intervention increased brain volume in both gray matter (anterior cingulate cortex, supplementary motor area, posterior middle frontal gyrus, and left superior temporal lobe) and white matter (anterior third of corpus callosum) [88]. In addition, Colcombe and colleagues showed that older adults with higher cardiovascular fitness levels are better at activating attentional resources, including decreased activation of the anterior cingulated cortex. One of the possible mechanisms by which physical activity may benefit cognition is that physical activity maintains brain plasticity, increases brain volume, stimulates neurogenesis and synaptogenesis, and increases neurotrophic factors in different areas of the brain, possibly providing reserve against later cognitive decline and dementia [89, 90].
- Basso JC, Suzuki WA (March 2017). "The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review". Brain Plasticity. 2 (2): 127–152. doi:10.3233/BPL-160040. PMC 5928534. PMID 29765853. Lay summary – Can A Single Exercise Session Benefit Your Brain? (12 June 2017).
A large collection of research in humans has shown that a single bout of exercise alters behavior at the level of affective state and cognitive functioning in several key ways. In terms of affective state, acute exercise decreases negative affect, increases positive affect, and decreases the psychological and physiological response to acute stress [28]. These effects have been reported to persist for up to 24 hours after exercise cessation [28, 29, 53]. In terms of cognitive functioning, acute exercise primarily enhances executive functions dependent on the prefrontal cortex including attention, working memory, problem solving, cognitive flexibility, verbal fluency, decision making, and inhibitory control [9]. These positive changes have been demonstrated to occur with very low to very high exercise intensities [9], with effects lasting for up to two hours after the end of the exercise bout (Fig. 1A) [27]. Moreover, many of these neuropsychological assessments measure several aspects of behavior including both accuracy of performance and speed of processing. McMorris and Hale performed a meta-analysis examining the effects of acute exercise on both accuracy and speed of processing, revealing that speed significantly improved post-exercise, with minimal or no effect on accuracy [17]. These authors concluded that increasing task difficulty or complexity may help to augment the effect of acute exercise on accuracy. ... However, in a comprehensive meta-analysis, Chang and colleagues found that exercise intensities ranging from very light (<50% MHR) to very hard (>93% MHR) have all been reported to improve cognitive functioning [9].
- Cunha GS, Ribeiro JL, Oliveira AR (June 2008). "[Levels of beta-endorphin in response to exercise and overtraining]". Arq Bras Endocrinol Metabol (in Portuguese). 52 (4): 589–598. doi:10.1590/S0004-27302008000400004. PMID 18604371.
Interestingly, some symptoms of OT are related to beta-endorphin (beta-end(1-31)) effects. Some of its effects, such as analgesia, increasing lactate tolerance, and exercise-induced euphoria, are important for training.
- Boecker H, Sprenger T, Spilker ME, Henriksen G, Koppenhoefer M, Wagner KJ, Valet M, Berthele A, Tolle TR (2008). "The runner's high: opioidergic mechanisms in the human brain". Cereb. Cortex. 18 (11): 2523–2531. doi:10.1093/cercor/bhn013. PMID 18296435.
The runner's high describes a euphoric state resulting from long-distance running.
- Josefsson T, Lindwall M, Archer T (2014). "Physical exercise intervention in depressive disorders: meta-analysis and systematic review". Scand J Med Sci Sports. 24 (2): 259–272. doi:10.1111/sms.12050. PMID 23362828.
- Rosenbaum S, Tiedemann A, Sherrington C, Curtis J, Ward PB (2014). "Physical activity interventions for people with mental illness: a systematic review and meta-analysis". J Clin Psychiatry. 75 (9): 964–974. doi:10.4088/JCP.13r08765. PMID 24813261.
This systematic review and meta-analysis found that physical activity reduced depressive symptoms among people with a psychiatric illness. The current meta-analysis differs from previous studies, as it included participants with depressive symptoms with a variety of psychiatric diagnoses (except dysthymia and eating disorders). ... This review provides strong evidence for the antidepressant effect of physical activity; however, the optimal exercise modality, volume, and intensity remain to be determined. ...
Conclusion
Few interventions exist whereby patients can hope to achieve improvements in both psychiatric symptoms and physical health simultaneously without significant risks of adverse effects. Physical activity offers substantial promise for improving outcomes for people living with mental illness, and the inclusion of physical activity and exercise programs within treatment facilities is warranted given the results of this review. - Szuhany KL, Bugatti M, Otto MW (October 2014). "A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor". J Psychiatr Res. 60C: 56–64. doi:10.1016/j.jpsychires.2014.10.003. PMC 4314337. PMID 25455510.
Consistent evidence indicates that exercise improves cognition and mood, with preliminary evidence suggesting that brain-derived neurotrophic factor (BDNF) may mediate these effects. The aim of the current meta-analysis was to provide an estimate of the strength of the association between exercise and increased BDNF levels in humans across multiple exercise paradigms. We conducted a meta-analysis of 29 studies (N = 1111 participants) examining the effect of exercise on BDNF levels in three exercise paradigms: (1) a single session of exercise, (2) a session of exercise following a program of regular exercise, and (3) resting BDNF levels following a program of regular exercise. Moderators of this effect were also examined. Results demonstrated a moderate effect size for increases in BDNF following a single session of exercise (Hedges' g = 0.46, p < 0.001). Further, regular exercise intensified the effect of a session of exercise on BDNF levels (Hedges' g = 0.59, p = 0.02). Finally, results indicated a small effect of regular exercise on resting BDNF levels (Hedges' g = 0.27, p = 0.005). ... Effect size analysis supports the role of exercise as a strategy for enhancing BDNF activity in humans.
- Lees C, Hopkins J (2013). "Effect of aerobic exercise on cognition, academic achievement, and psychosocial function in children: a systematic review of randomized control trials". Prev Chronic Dis. 10: E174. doi:10.5888/pcd10.130010. PMC 3809922. PMID 24157077.
This omission is relevant, given the evidence that aerobic-based physical activity generates structural changes in the brain, such as neurogenesis, angiogenesis, increased hippocampal volume, and connectivity (12,13). In children, a positive relationship between aerobic fitness, hippocampal volume, and memory has been found (12,13). ... Mental health outcomes included reduced depression and increased self-esteem, although no change was found in anxiety levels (18). ... This systematic review of the literature found that [aerobic physical activity (APA)] is positively associated with cognition, academic achievement, behavior, and psychosocial functioning outcomes. Importantly, Shephard also showed that curriculum time reassigned to APA still results in a measurable, albeit small, improvement in academic performance (24). ... The actual aerobic-based activity does not appear to be a major factor; interventions used many different types of APA and found similar associations. In positive association studies, intensity of the aerobic activity was moderate to vigorous. The amount of time spent in APA varied significantly between studies; however, even as little as 45 minutes per week appeared to have a benefit.
- Mura G, Moro MF, Patten SB, Carta MG (2014). "Exercise as an add-on strategy for the treatment of major depressive disorder: a systematic review". CNS Spectr. 19 (6): 496–508. doi:10.1017/S1092852913000953. PMID 24589012.
Considered overall, the studies included in the present review showed a strong effectiveness of exercise combined with antidepressants. ...
Conclusions
This is the first review to have focused on exercise as an add-on strategy in the treatment of MDD. Our findings corroborate some previous observations that were based on few studies and which were difficult to generalize.41,51,73,92,93 Given the results of the present article, it seems that exercise might be an effective strategy to enhance the antidepressant effect of medication treatments. Moreover, we hypothesize that the main role of exercise on treatment-resistant depression is in inducing neurogenesis by increasing BDNF expression, as was demonstrated by several recent studies. - Box 1: Patients with Depression Who May Particularly Benefit From Exercise Programs
Box 2: Depressive Disorders Other Than Major Depression That May Benefit From Exercise Programs
Box 3: The Characteristics of an Exercise Program that will Maximize the Anti-depressive Properties - Den Heijer AE, Groen Y, Tucha L, Fuermaier AB, Koerts J, Lange KW, Thome J, Tucha O (July 2016). "Sweat it out? The effects of physical exercise on cognition and behavior in children and adults with ADHD: a systematic literature review". J. Neural Transm. (Vienna). 124 (Suppl 1): 3–26. doi:10.1007/s00702-016-1593-7. PMC 5281644. PMID 27400928.
- Kamp CF, Sperlich B, Holmberg HC (July 2014). "Exercise reduces the symptoms of attention-deficit/hyperactivity disorder and improves social behaviour, motor skills, strength and neuropsychological parameters". Acta Paediatr. 103 (7): 709–14. doi:10.1111/apa.12628. PMID 24612421.
The present review summarises the impact of exercise interventions (1–10 weeks in duration with at least two sessions each week) on parameters related to ADHD in 7-to 13-year-old children. We may conclude that all different types of exercise (here yoga, active games with and without the involvement of balls, walking and athletic training) attenuate the characteristic symptoms of ADHD and improve social behaviour, motor skills, strength and neuropsychological parameters without any undesirable side effects. Available reports do not reveal which type, intensity, duration and frequency of exercise is most effective in this respect and future research focusing on this question with randomised and controlled long-term interventions is warranted.
- Petersen RC, Lopez O, Armstrong MJ, Getchius T, Ganguli M, Gloss D, Gronseth GS, Marson D, Pringsheim T, Day GS, Sager M, Stevens J, Rae-Grant A (January 2018). "Practice guideline update summary: Mild cognitive impairment – Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology". Neurology. Special article. 90 (3): 126–135. doi:10.1212/WNL.0000000000004826. PMC 5772157. PMID 29282327. Lay summary – Exercise may improve thinking ability and memory (27 December 2017).
In patients with MCI, exercise training (6 months) is likely to improve cognitive measures and cognitive training may improve cognitive measures. ... Clinicians should recommend regular exercise (Level B). ... Recommendation
For patients diagnosed with MCI, clinicians should recommend regular exercise (twice/week) as part of an overall approach to management (Level B). - Farina N, Rusted J, Tabet N (January 2014). "The effect of exercise interventions on cognitive outcome in Alzheimer's disease: a systematic review". Int Psychogeriatr. 26 (1): 9–18. doi:10.1017/S1041610213001385. PMID 23962667.
Six RCTs were identified that exclusively considered the effect of exercise in AD patients. Exercise generally had a positive effect on rate of cognitive decline in AD. A meta-analysis found that exercise interventions have a positive effect on global cognitive function, 0.75 (95% CI = 0.32–1.17). ... The most prevalent subtype of dementia is Alzheimer’s disease (AD), accounting for up to 65.0% of all dementia cases ... Cognitive decline in AD is attributable at least in part to the buildup of amyloid and tau proteins, which promote neuronal dysfunction and death (Hardy and Selkoe, 2002; Karran et al., 2011). Evidence in transgenic mouse models of AD, in which the mice have artificially elevated amyloid load, suggests that exercise programs are able to improve cognitive function (Adlard et al., 2005; Nichol et al., 2007). Adlard and colleagues also determined that the improvement in cognitive performance occurred in conjunction with a reduced amyloid load. Research that includes direct indices of change in such biomarkers will help to determine the mechanisms by which exercise may act on cognition in AD.
- Rao AK, Chou A, Bursley B, Smulofsky J, Jezequel J (January 2014). "Systematic review of the effects of exercise on activities of daily living in people with Alzheimer's disease". Am J Occup Ther. 68 (1): 50–56. doi:10.5014/ajot.2014.009035. PMC 5360200. PMID 24367955.
Alzheimer’s disease (AD) is a progressive neurological disorder characterized by loss in cognitive function, abnormal behavior, and decreased ability to perform basic activities of daily living [(ADLs)] ... All studies included people with AD who completed an exercise program consisting of aerobic, strength, or balance training or any combination of the three. The length of the exercise programs varied from 12 weeks to 12 months. ... Six studies involving 446 participants tested the effect of exercise on ADL performance ... exercise had a large and significant effect on ADL performance (z = 4.07, p < .0001; average effect size = 0.80). ... These positive effects were apparent with programs ranging in length from 12 wk (Santana-Sosa et al., 2008; Teri et al., 2003) and intermediate length of 16 wk (Roach et al., 2011; Vreugdenhil et al., 2012) to 6 mo (Venturelli et al., 2011) and 12 mo (Rolland et al., 2007). Furthermore, the positive effects of a 3-mo intervention lasted 24 mo (Teri et al., 2003). ... No adverse effects of exercise on ADL performance were noted. ... The study with the largest effect size implemented a walking and aerobic program of only 30 min four times a week (Venturelli et al., 2011).
- Mattson MP (2014). "Interventions that improve body and brain bioenergetics for Parkinson's disease risk reduction and therapy". J Parkinsons Dis. 4 (1): 1–13. doi:10.3233/JPD-130335. PMID 24473219.
- Grazina R, Massano J (2013). "Physical exercise and Parkinson's disease: influence on symptoms, disease course and prevention". Rev Neurosci. 24 (2): 139–152. doi:10.1515/revneuro-2012-0087. PMID 23492553.
- van der Kolk NM, King LA (September 2013). "Effects of exercise on mobility in people with Parkinson's disease". Mov. Disord. 28 (11): 1587–1596. doi:10.1002/mds.25658. PMID 24132847.
- Tomlinson CL, Patel S, Meek C, Herd CP, Clarke CE, Stowe R, Shah L, Sackley CM, Deane KH, Wheatley K, Ives N (September 2013). "Physiotherapy versus placebo or no intervention in Parkinson's disease". Cochrane Database Syst Rev. 9 (9): CD002817. doi:10.1002/14651858.CD002817.pub4. PMID 24018704.
- Blondell SJ, Hammersley-Mather R, Veerman JL (May 2014). "Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies". BMC Public Health. 14: 510. doi:10.1186/1471-2458-14-510. PMC 4064273. PMID 24885250.
Longitudinal observational studies show an association between higher levels of physical activity and a reduced risk of cognitive decline and dementia. A case can be made for a causal interpretation. Future research should use objective measures of physical activity, adjust for the full range of confounders and have adequate follow-up length. Ideally, randomised controlled trials will be conducted. ... On the whole the results do, however, lend support to the notion of a causal relationship between physical activity, cognitive decline and dementia, according to the established criteria for causal inference.
- Carroll ME, Smethells JR (February 2016). "Sex Differences in Behavioral Dyscontrol: Role in Drug Addiction and Novel Treatments". Front. Psychiatry. 6: 175. doi:10.3389/fpsyt.2015.00175. PMC 4745113. PMID 26903885.
There is accelerating evidence that physical exercise is a useful treatment for preventing and reducing drug addiction ... In some individuals, exercise has its own rewarding effects, and a behavioral economic interaction may occur, such that physical and social rewards of exercise can substitute for the rewarding effects of drug abuse. ... The value of this form of treatment for drug addiction in laboratory animals and humans is that exercise, if it can substitute for the rewarding effects of drugs, could be self-maintained over an extended period of time. Work to date in [laboratory animals and humans] regarding exercise as a treatment for drug addiction supports this hypothesis. ... However, a RTC study was recently reported by Rawson et al. (226), whereby they used 8 weeks of exercise as a post-residential treatment for METH addiction, showed a significant reduction in use (confirmed by urine screens) in participants who had been using meth 18 days or less a month. ... Animal and human research on physical exercise as a treatment for stimulant addiction indicates that this is one of the most promising treatments on the horizon. [emphasis added]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA (September 2013). "Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis". Neurosci Biobehav Rev. 37 (8): 1622–1644. doi:10.1016/j.neubiorev.2013.06.011. PMC 3788047. PMID 23806439.
- Olsen CM (December 2011). "Natural rewards, neuroplasticity, and non-drug addictions". Neuropharmacology. 61 (7): 1109–1122. doi:10.1016/j.neuropharm.2011.03.010. PMC 3139704. PMID 21459101.
Similar to environmental enrichment, studies have found that exercise reduces self-administration and relapse to drugs of abuse (Cosgrove et al., 2002; Zlebnik et al., 2010). There is also some evidence that these preclinical findings translate to human populations, as exercise reduces withdrawal symptoms and relapse in abstinent smokers (Daniel et al., 2006; Prochaska et al., 2008), and one drug recovery program has seen success in participants that train for and compete in a marathon as part of the program (Butler, 2005). ... In humans, the role of dopamine signaling in incentive-sensitization processes has recently been highlighted by the observation of a dopamine dysregulation syndrome in some patients taking dopaminergic drugs. This syndrome is characterized by a medication-induced increase in (or compulsive) engagement in non-drug rewards such as gambling, shopping, or sex (Evans et al., 2006; Aiken, 2007; Lader, 2008).
- Linke SE, Ussher M (2015). "Exercise-based treatments for substance use disorders: evidence, theory, and practicality". Am J Drug Alcohol Abuse. 41 (1): 7–15. doi:10.3109/00952990.2014.976708. PMC 4831948. PMID 25397661.
The limited research conducted suggests that exercise may be an effective adjunctive treatment for SUDs. In contrast to the scarce intervention trials to date, a relative abundance of literature on the theoretical and practical reasons supporting the investigation of this topic has been published. ... numerous theoretical and practical reasons support exercise-based treatments for SUDs, including psychological, behavioral, neurobiological, nearly universal safety profile, and overall positive health effects.
- Zhou Y, Zhao M, Zhou C, Li R (July 2015). "Sex differences in drug addiction and response to exercise intervention: From human to animal studies". Front. Neuroendocrinol. 40: 24–41. doi:10.1016/j.yfrne.2015.07.001. PMC 4712120. PMID 26182835.
Collectively, these findings demonstrate that exercise may serve as a substitute or competition for drug abuse by changing ΔFosB or cFos immunoreactivity in the reward system to protect against later or previous drug use. ... As briefly reviewed above, a large number of human and rodent studies clearly show that there are sex differences in drug addiction and exercise. The sex differences are also found in the effectiveness of exercise on drug addiction prevention and treatment, as well as underlying neurobiological mechanisms. The postulate that exercise serves as an ideal intervention for drug addiction has been widely recognized and used in human and animal rehabilitation. ... In particular, more studies on the neurobiological mechanism of exercise and its roles in preventing and treating drug addiction are needed.
- Cormie P, Nowak AK, Chambers SK, Galvão DA, Newton RU (April 2015). "The potential role of exercise in neuro-oncology". Front. Oncol. 5: 85. doi:10.3389/fonc.2015.00085. PMC 4389372. PMID 25905043.
- Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, McMurdo M, Mead GE (September 2013). "Exercise for depression". Cochrane Database Syst. Rev. 9 (9): CD004366. doi:10.1002/14651858.CD004366.pub6. PMID 24026850.
Exercise is moderately more effective than a control intervention for reducing symptoms of depression, but analysis of methodologically robust trials only shows a smaller effect in favour of exercise. When compared to psychological or pharmacological therapies, exercise appears to be no more effective, though this conclusion is based on a few small trials.
- Brené S, Bjørnebekk A, Aberg E, Mathé AA, Olson L, Werme M (2007). "Running is rewarding and antidepressive". Physiol. Behav. 92 (1–2): 136–140. doi:10.1016/j.physbeh.2007.05.015. PMC 2040025. PMID 17561174.
- Gong H, Ni C, Shen X, Wu T, Jiang C (February 2015). "Yoga for prenatal depression: a systematic review and meta-analysis". BMC Psychiatry. 15: 14. doi:10.1186/s12888-015-0393-1. PMC 4323231. PMID 25652267.
- Miller KJ, Gonçalves-Bradley DC, Areerob P, Hennessy D, Mesagno C, Grace F (2020). "Comparative effectiveness of three exercise types to treat clinical depression in older adults: A systematic review and network meta-analysis of randomised controlled trials". Ageing Research Reviews. 58: 100999. doi:10.1016/j.arr.2019.100999. PMID 31837462.
- Tantimonaco M, Ceci R, Sabatini S, Catani MV, Rossi A, Gasperi V, Maccarrone M (2014). "Physical activity and the endocannabinoid system: an overview". Cell. Mol. Life Sci. 71 (14): 2681–98. doi:10.1007/s00018-014-1575-6. PMID 24526057.
- Dinas PC, Koutedakis Y, Flouris AD (2011). "Effects of exercise and physical activity on depression". Ir J Med Sci. 180 (2): 319–25. doi:10.1007/s11845-010-0633-9. PMID 21076975.
- Szabo A, Billett E, Turner J (2001). "Phenylethylamine, a possible link to the antidepressant effects of exercise?". Br J Sports Med. 35 (5): 342–43. doi:10.1136/bjsm.35.5.342. PMC 1724404. PMID 11579070.
- Lindemann L, Hoener MC (2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends Pharmacol. Sci. 26 (5): 274–81. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- Berry MD (2007). "The potential of trace amines and their receptors for treating neurological and psychiatric diseases". Rev Recent Clin Trials. 2 (1): 3–19. doi:10.2174/157488707779318107. PMID 18473983. S2CID 7127324.
- Yang, PY; Ho, KH; Chen, HC; Chien, MY (2012). "Exercise training improves sleep quality in middle-aged and older adults with sleep problems: A systematic review". Journal of Physiotherapy. 58 (3): 157–63. doi:10.1016/S1836-9553(12)70106-6. PMID 22884182.
- Buman, M.P.; King, A.C. (2010). "Exercise as a Treatment to Enhance Sleep". American Journal of Lifestyle Medicine. 31 (5): 514. doi:10.1177/1559827610375532.
- Banno, M; Harada, Y; Taniguchi, M; Tobita, R; Tsujimoto, H; Tsujimoto, Y; Kataoka, Y; Noda, A (2018). "Exercise can improve sleep quality: a systematic review and meta-analysis". PeerJ. 6: e5172. doi:10.7717/peerj.5172. PMC 6045928. PMID 30018855.
- Lorenz, TA; Meston, CM (2013). "Acute Exercise Improves Physical Sexual Arousal in Women Taking Antidepressants". Annals of Behavioral Medicine. 43 (3): 352–361. doi:10.1007/s12160-011-9338-1. PMC 3422071. PMID 22403029.
- Brook MS, Wilkinson DJ, Phillips BE, Perez-Schindler J, Philp A, Smith K, Atherton PJ (January 2016). "Skeletal muscle homeostasis and plasticity in youth and ageing: impact of nutrition and exercise". Acta Physiologica. 216 (1): 15–41. doi:10.1111/apha.12532. PMC 4843955. PMID 26010896.
- Phillips SM (May 2014). "A brief review of critical processes in exercise-induced muscular hypertrophy". Sports Med. 44 Suppl 1: S71–S77. doi:10.1007/s40279-014-0152-3. PMC 4008813. PMID 24791918.
- Brioche T, Pagano AF, Py G, Chopard A (April 2016). "Muscle wasting and aging: Experimental models, fatty infiltrations, and prevention". Molecular Aspects of Medicine. 50: 56–87. doi:10.1016/j.mam.2016.04.006. PMID 27106402.
- Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, Etheridge T, Rathmacher JA, Smith K, Szewczyk NJ, Atherton PJ (June 2013). "Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism" (PDF). J. Physiol. 591 (11): 2911–23. doi:10.1113/jphysiol.2013.253203. PMC 3690694. PMID 23551944. Retrieved 27 May 2016.
- Wilkinson DJ, Hossain T, Limb MC, Phillips BE, Lund J, Williams JP, Brook MS, Cegielski J, Philp A, Ashcroft S, Rathmacher JA, Szewczyk NJ, Smith K, Atherton PJ (2018). "Impact of the calcium form of β-hydroxy-β-methylbutyrate upon human skeletal muscle protein metabolism". Clinical Nutrition (Edinburgh, Scotland). 37 (6): 2068–2075. doi:10.1016/j.clnu.2017.09.024. PMC 6295980. PMID 29097038.
Ca-HMB led a significant and rapid (<60 min) peak in plasma HMB concentrations (483.6 ± 14.2 μM, p < 0.0001). This rise in plasma HMB was accompanied by increases in MPS (PA: 0.046 ± 0.004%/h, CaHMB: 0.072 ± 0.004%/h, p < [0.001]) and suppressions in MPB (PA: 7.6 ± 1.2 μmol Phe per leg min−1, Ca-HMB: 5.2 ± 0.8 μmol Phe per leg min−1, p < 0.01). ... During the first 2.5 h period we gathered postabsorptive/fasted measurements, the volunteers then consumed 3.42 g of Ca-HMB (equivalent to 2.74 g of FA-HMB) ... It may seem difficult for one to reconcile that acute provision of CaHMB, in the absence of exogenous nutrition (i.e. EAA's) and following an overnight fast, is still able to elicit a robust, perhaps near maximal stimulation of MPS, i.e. raising the question as to where the additional AA's substrates required for supporting this MPS response are coming from. It would appear that the AA's to support this response are derived from endogenous intracellular/plasma pools and/or protein breakdown (which will increase in fasted periods). ... To conclude, a large single oral dose (~3 g) of Ca-HMB robustly (near maximally) stimulates skeletal muscle anabolism, in the absence of additional nutrient intake; the anabolic effects of Ca-HMB are equivalent to FA-HMB, despite purported differences in bioavailability (Fig. 4).
- Phillips SM (July 2015). "Nutritional supplements in support of resistance exercise to counter age-related sarcopenia". Adv. Nutr. 6 (4): 452–60. doi:10.3945/an.115.008367. PMC 4496741. PMID 26178029.
- Adaptation of mitochondrial ATP-production in human skeletal muscle to endurance training and detraining
- Boushel R, Lundby C, Qvortrup K, Sahlin K (October 2014). "Mitochondrial plasticity with exercise training and extreme environments". Exerc. Sport Sci. Rev. 42 (4): 169–74. doi:10.1249/JES.0000000000000025. PMID 25062000.
- Valero T (2014). "Mitochondrial biogenesis: pharmacological approaches". Curr. Pharm. Des. 20 (35): 5507–09. doi:10.2174/138161282035140911142118. hdl:10454/13341. PMID 24606795.
- Lipton JO, Sahin M (October 2014). "The neurology of mTOR". Neuron. 84 (2): 275–91. doi:10.1016/j.neuron.2014.09.034. PMC 4223653. PMID 25374355.
Figure 2: The mTOR Signaling Pathway - Wang, E; Næss, MS; Hoff, J; Albert, TL; Pham, Q; Richardson, RS; Helgerud, J (16 November 2013). "Exercise-training-induced changes in metabolic capacity with age: the role of central cardiovascular plasticity". Age (Dordrecht, Netherlands). 36 (2): 665–76. doi:10.1007/s11357-013-9596-x. PMC 4039249. PMID 24243396.
- Potempa, K; Lopez, M; Braun, LT; Szidon, JP; Fogg, L; Tincknell, T (January 1995). "Physiological outcomes of aerobic exercise training in hemiparetic stroke patients". Stroke: A Journal of Cerebral Circulation. 26 (1): 101–05. doi:10.1161/01.str.26.1.101. PMID 7839377.
- Wilmore, JH; Stanforth, PR; Gagnon, J; Leon, AS; Rao, DC; Skinner, JS; Bouchard, C (July 1996). "Endurance exercise training has a minimal effect on resting heart rate: the HERITAGE Study". Medicine & Science in Sports & Exercise. 28 (7): 829–35. doi:10.1097/00005768-199607000-00009. PMID 8832536.
- Carter, JB; Banister, EW; Blaber, AP (2003). "Effect of endurance exercise on autonomic control of heart rate". Sports Medicine. 33 (1): 33–46. doi:10.2165/00007256-200333010-00003. PMID 12477376.
- Chen, Chao‐Yin; Dicarlo, Stephen E. (January 1998). "Endurance exercise training‐induced resting Bradycardia: A brief review". Sports Medicine, Training and Rehabilitation. 8 (1): 37–77. doi:10.1080/15438629709512518.
- Crewther, BT; Heke, TL; Keogh, JW (February 2013). "The effects of a resistance-training program on strength, body composition and baseline hormones in male athletes training concurrently for rugby union 7's". The Journal of Sports Medicine and Physical Fitness. 53 (1): 34–41. PMID 23470909.
- Schoenfeld, BJ (June 2013). "Postexercise hypertrophic adaptations: a reexamination of the hormone hypothesis and its applicability to resistance training program design". Journal of Strength and Conditioning Research. 27 (6): 1720–30. doi:10.1519/JSC.0b013e31828ddd53. PMID 23442269. S2CID 25068522.
- Dalgas, U; Stenager, E; Lund, C; Rasmussen, C; Petersen, T; Sørensen, H; Ingemann-Hansen, T; Overgaard, K (July 2013). "Neural drive increases following resistance training in patients with multiple sclerosis". Journal of Neurology. 260 (7): 1822–32. doi:10.1007/s00415-013-6884-4. PMID 23483214.
- Staron, RS; Karapondo, DL; Kraemer, WJ; Fry, AC; Gordon, SE; Falkel, JE; Hagerman, FC; Hikida, RS (March 1994). "Skeletal muscle adaptations during early phase of heavy-resistance training in men and women". Journal of Applied Physiology. 76 (3): 1247–55. doi:10.1152/jappl.1994.76.3.1247. PMID 8005869.
- Folland, JP; Williams, AG (2007). "The adaptations to strength training : morphological and neurological contributions to increased strength". Sports Medicine. 37 (2): 145–68. doi:10.2165/00007256-200737020-00004. PMID 17241104.
- Moritani, T; deVries, HA (June 1979). "Neural factors versus hypertrophy in the time course of muscle strength gain". American Journal of Physical Medicine. 58 (3): 115–30. PMID 453338.
- Narici, MV; Roi, GS; Landoni, L; Minetti, AE; Cerretelli, P (1989). "Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps". European Journal of Applied Physiology and Occupational Physiology. 59 (4): 310–09. doi:10.1007/bf02388334. PMID 2583179.
- Pedersen, BK (July 2013). "Muscle as a secretory organ". Comprehensive Physiology. 3 (3): 1337–62. doi:10.1002/cphy.c120033. ISBN 978-0-470-65071-4. PMID 23897689.
- Cohen S, Williamson GM (1991). "Stress and infectious disease in humans". Psychological Bulletin. 109 (1): 5–24. doi:10.1037/0033-2909.109.1.5. PMID 2006229.
- Borer KT, Wuorinen EC, Lukos JR, Denver JW, Porges SW, Burant CF (August 2009). "Two bouts of exercise before meals but not after meals, lower fasting blood glucose". Medicine and Science in Sports and Exercise. 41 (8): 1606–14. doi:10.1249/MSS.0b013e31819dfe14. PMID 19568199.
- Wisløff U, Ellingsen Ø, Kemi OJ (July 2009). "High=Intensity Interval Training to Maximize Cardiac Benefit of Exercise Taining?". Exercise and Sport Sciences Reviews. 37 (3): 139–46. doi:10.1097/JES.0b013e3181aa65fc. PMID 19550205. S2CID 25057561.
- Paillard T, Rolland Y, de Souto Barreto P (July 2015). "Protective Effects of Physical Exercise in Alzheimer's Disease and Parkinson's Disease: A Narrative Review". J Clin Neurol. 11 (3): 212–219. doi:10.3988/jcn.2015.11.3.212. PMC 4507374. PMID 26174783.
Aerobic physical exercise (PE) activates the release of neurotrophic factors and promotes angiogenesis, thereby facilitating neurogenesis and synaptogenesis, which in turn improve memory and cognitive functions. ... Exercise limits the alteration in dopaminergic neurons in the substantia nigra and contributes to optimal functioning of the basal ganglia involved in motor commands and control by adaptive mechanisms involving dopamine and glutamate neurotransmission.
- Szuhany KL, Bugatti M, Otto MW (January 2015). "A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor". Journal of Psychiatric Research. 60: 56–64. doi:10.1016/j.jpsychires.2014.10.003. PMC 4314337. PMID 25455510.
Consistent evidence indicates that exercise improves cognition and mood, with preliminary evidence suggesting that brain-derived neurotrophic factor (BDNF) may mediate these effects. The aim of the current meta-analysis was to provide an estimate of the strength of the association between exercise and increased BDNF levels in humans across multiple exercise paradigms. We conducted a meta-analysis of 29 studies (N = 1111 participants) examining the effect of exercise on BDNF levels in three exercise paradigms: (1) a single session of exercise, (2) a session of exercise following a program of regular exercise, and (3) resting BDNF levels following a program of regular exercise. Moderators of this effect were also examined. Results demonstrated a moderate effect size for increases in BDNF following a single session of exercise (Hedges' g = 0.46, p < 0.001). Further, regular exercise intensified the effect of a session of exercise on BDNF levels (Hedges' g = 0.59, p = 0.02). Finally, results indicated a small effect of regular exercise on resting BDNF levels (Hedges' g = 0.27, p = 0.005). ... Effect size analysis supports the role of exercise as a strategy for enhancing BDNF activity in humans
- Bouchard J, Villeda SA (2015). "Aging and brain rejuvenation as systemic events". J. Neurochem. 132 (1): 5–19. doi:10.1111/jnc.12969. PMC 4301186. PMID 25327899.
From a molecular perspective, elevated systemic levels of circulating growth factors such as vascular endothelial growth factor and insulin-like growth factor 1 (IGF-1) in blood elicited by increased exercise have been shown to mediate, in part, enhancements in neurogenesis (Trejo et al. 2001; Fabel et al. 2003).
- Silverman MN, Deuster PA (October 2014). "Biological mechanisms underlying the role of physical fitness in health and resilience". Interface Focus. 4 (5): 20140040. doi:10.1098/rsfs.2014.0040. PMC 4142018. PMID 25285199.
Importantly, physical exercise can improve growth factor signalling directly or indirectly by reducing pro-inflammatory signalling [33]. Exercise-induced increases in brain monoamines (norepinephrine and serotonin) may also contribute to increased expression of hippocampal BDNF [194]. In addition, other growth factors—insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor—have been shown to play an important role in BDNF-induced effects on neuroplasticity [33,172,190,192], as well as exerting neuroprotective effects of their own [33,214,215], thereby contributing to the beneficial effects of exercise on brain health.
- Gomez-Pinilla F, Hillman C (January 2013). "The influence of exercise on cognitive abilities". Compr. Physiol. 3 (1): 403–28. doi:10.1002/cphy.c110063. ISBN 978-0-470-65071-4. PMC 3951958. PMID 23720292.
Abundant research in the last decade has shown that exercise is one of the strongest promoters of neurogenesis in the brain of adult rodents (97, 102) and humans (1,61), and this has introduced the possibility that proliferating neurons could contribute to the cognitive enhancement observed with exercise. In addition to BDNF, the actions of IGF-1 and vascular endothelial growth factor (VEGF) (54) are considered essential for the angiogenic and neurogenic effects of exercise in the brain. Although the action of exercise on brain angiogenesis has been known for many years (10), it is not until recently that neurovascular adaptations in the hippocampus have been associated with cognitive function (29). Exercise enhances the proliferation of brain endothelial cells throughout the brain (113), hippocampal IGF gene expression (47), and serum levels of both IGF (178) and VEGF (63). IGF-1 and VEGF, apparently produced in the periphery, support exercise induced neurogenesis and angiogenesis, as corroborated by blocking the effects of exercise using antibodies against IGF-1 (47) or VEGF (63).
- Tarumi T, Zhang R (January 2014). "Cerebral hemodynamics of the aging brain: risk of Alzheimer disease and benefit of aerobic exercise". Front Physiol. 5: 6. doi:10.3389/fphys.2014.00006. PMC 3896879. PMID 24478719.
Exercise-related improvements in brain function and structure may be conferred by the concurrent adaptations in vascular function and structure. Aerobic exercise increases the peripheral levels of growth factors (e.g., BDNF, IFG-1, and VEGF) which cross the blood-brain barrier (BBB) and stimulate neurogenesis and angiogenesis (Trejo et al., 2001; Lee et al., 2002; Fabel et al., 2003; Lopez-Lopez et al., 2004). Consistent with this, exercise-related enlargement of hippocampus was accompanied by increases in cerebral blood volume and capillary densities (Pereira et al., 2007). Enhanced cerebral perfusion may not only facilitate the delivery of energy substrates, but also lower the risk of vascular-related brain damages, including WMH and silent infarct (Tseng et al., 2013). Furthermore, regular aerobic exercise is associated with lower levels of Aβ deposition in individuals with APOE4 positive (Head et al., 2012), which may also reduce the risk of cerebral amyloid angiopathy and microbleeds (Poels et al., 2010).
- Baker, Philip R.A.; Francis, Daniel P.; Soares, Jesus; Weightman, Alison L.; Foster, Charles (1 January 2015). "Community wide interventions for increasing physical activity". The Cochrane Database of Systematic Reviews. 1: CD008366. doi:10.1002/14651858.CD008366.pub3. PMID 25556970. S2CID 205194633.
- Howe, Tracey E; Rochester, Lynn; Neil, Fiona; Skelton, Dawn A; Ballinger, Claire (9 November 2011). Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd. pp. CD004963. doi:10.1002/14651858.cd004963.pub3. PMID 22071817. S2CID 205176433.
- Liu, Chiung-ju; Latham, Nancy K (8 July 2009). Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd. pp. CD002759. doi:10.1002/14651858.cd002759.pub2. PMC 4324332. PMID 19588334.
- Gc, V; Wilson, EC; Suhrcke, M; Hardeman, W; Sutton, S; VBI Programme, Team (April 2016). "Are brief interventions to increase physical activity cost-effective? A systematic review". British Journal of Sports Medicine. 50 (7): 408–17. doi:10.1136/bjsports-2015-094655. PMC 4819643. PMID 26438429.
- Kahn EB, Ramsey LT, Brownson RC, Heath GW, Howze EH, Powell KE, Stone EJ, Rajab MW, Corso P (May 2002). "The effectiveness of interventions to increase physical activity. A systematic review". Am J Prev Med. 22 (4 Suppl): 73–107. doi:10.1016/S0749-3797(02)00434-8. PMID 11985936.
- Durán, Víctor Hugo. "Stopping the rising tide of chronic diseases Everyone's Epidemic". Pan American Health Organization. paho.org. Retrieved 10 January 2009.
- Dons, E (2018). "Transport mode choice and body mass index: Cross-sectional and longitudinal evidence from a European-wide study". Environment International. 119 (119): 109–16. doi:10.1016/j.envint.2018.06.023. hdl:10044/1/61061. PMID 29957352.
- Baker, Philip RA; Dobbins, Maureen; Soares, Jesus; Francis, Daniel P; Weightman, Alison L; Costello, Joseph T (6 January 2015). "Public health interventions for increasing physical activity in children, adolescents and adults: an overview of systematic reviews" (PDF). Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd. doi:10.1002/14651858.cd011454.
- Reed, Jennifer L; Prince, Stephanie A; Cole, Christie A; Fodor, J; Hiremath, Swapnil; Mullen, Kerri-Anne; Tulloch, Heather E; Wright, Erica; Reid, Robert D (19 December 2014). "Workplace physical activity interventions and moderate-to-vigorous intensity physical activity levels among working-age women: a systematic review protocol". Systematic Reviews. 3 (1): 147. doi:10.1186/2046-4053-3-147. PMC 4290810. PMID 25526769.
- Laeremans, M (2018). "Black Carbon Reduces the Beneficial Effect of Physical Activity on Lung Function". Medicine & Science in Sports & Exercise. 50 (9): 1875–1881. doi:10.1249/MSS.0000000000001632. hdl:10044/1/63478. PMID 29634643.
- Xu, Huilan; Wen, Li Ming; Rissel, Chris (19 March 2015). "Associations of Parental Influences with Physical Activity and Screen Time among Young Children: A Systematic Review". Journal of Obesity. 2015: 546925. doi:10.1155/2015/546925. PMC 4383435. PMID 25874123.
- "Youth Physical Activity Guidelines". Centers for Disease Control and Prevention. 23 January 2019.
- "Health and Participation". ec.europa.eu. 25 June 2013. Archived from the original on 5 July 2019.
- "WHO: Obesity and overweight". World Health Organization. Archived from the original on 18 December 2008. Retrieved 10 January 2009.
- Kennedy AB, Resnick PB (May 2015). "Mindfulness and Physical Activity". American Journal of Lifestyle Medicine. 9 (3): 3221–23. doi:10.1177/1559827614564546.
- Hernandez, Javier (24 June 2008). "Car-Free Streets, a Colombian Export, Inspire Debate". NY Times. NY Times.
- Sullivan, Nicky. "Gyms". Travel Fish. Travel Fish. Retrieved 8 December 2016.
- Tatlow, Anita. "When in Sweden...making the most of the great outdoors!". Stockholm on a Shoestring. Stockholm on a Shoestring. Retrieved 5 December 2016.
- Langfitt, Frank. "Beijing's Other Games: Dancing In The Park". National Public Radio. National Public Radio. Retrieved 5 December 2016.
- Kimber N.; Heigenhauser G.; Spriet L.; Dyck D. (2003). "Skeletal muscle fat and carbohydrate metabolism during recovery from glycogen-depleting exercise in humans". The Journal of Physiology. 548 (3): 919–27. doi:10.1113/jphysiol.2002.031179. PMC 2342904. PMID 12651914.
- Reilly T, Ekblom B (June 2005). "The use of recovery methods post-exercise". J. Sports Sci. 23 (6): 619–27. doi:10.1080/02640410400021302. PMID 16195010.
- "How to Identify Overtraining Syndrome - 23 Warning Signs". Retrieved 30 May 2020.
- "Quotes About Exercise Top 10 List".
- "History of Fitness". www.unm.edu. Retrieved 20 September 2017.
- "physical culture". Encyclopedia Britannica. Retrieved 20 September 2017.
- Bogdanovic, Nikolai (19 December 2017). Fit to Fight: A History of the Royal Army Physical Training Corps 1860–2015. Bloomsbury USA. ISBN 978-1-4728-2421-9.
- Campbell, James D. (16 March 2016). 'The Army Isn't All Work': Physical Culture and the Evolution of the British Army, 1860–1920. Routledge. ISBN 978-1-317-04453-6.
- Mason, Tony; Riedi, Eliza (4 November 2010). Sport and the Military: The British Armed Forces 1880–1960. Cambridge University Press. ISBN 978-1-139-78897-7.
- "The Fitness League History". The Fitness League. Archived from the original on 29 July 2009. Retrieved 8 April 2015.
- Kuper, Simon (11 September 2009). "The man who invented exercise". Financial Times. Retrieved 12 September 2009.
- Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW (1953). "Coronary heart-disease and physical activity of work". Lancet. 262 (6795): 1053–57. doi:10.1016/S0140-6736(53)90665-5. PMID 13110049.
- Zhu, S.; Eclarinal, J.; Baker, M.S.; Li, G.; Waterland, R.A. (2016). "Developmental programming of energy balance regulation: is physical activity more "programmable" than food intake?". Proceedings of the Nutrition Society. 75 (1): 73–77. doi:10.1017/s0029665115004127. PMID 26511431.
- Acosta, W.; Meek, T.H.; Schutz, H.; Dlugosz, E.M.; Vu, K.T.; Garland Jr, T. (2015). "Effects of early-onset voluntary exercise on adult physical activity and associated phenotypes in mice". Physiology & Behavior. 149: 279–86. doi:10.1016/j.physbeh.2015.06.020. PMID 26079567.
- Swallow, John G; Carter, Patrick A; Garland, Jr, Theodore (1998). "Artificial selection for increased wheel-running behavior in house mice". Behavior Genetics. 28 (3): 227–37. doi:10.1023/A:1021479331779. PMID 9670598.
- Swallow, John G; Garland, Theodore; Carter, Patrick A; Zhan, Wen-Zhi; Sieck, Gary C (1998). "Effects of voluntary activity and genetic selection on aerobic capacity in house mice (Mus domesticus)". Journal of Applied Physiology. 84 (1): 69–76. doi:10.1152/jappl.1998.84.1.69. PMID 9451619.
- Rhodes, J.S.; Van Praag, H; Jeffrey, S; Girard, I; Mitchell, G.S.; Garland Jr, T; Gage, F.H. (2003). "Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running". Behavioral Neuroscience. 117 (5): 1006–16. doi:10.1037/0735-7044.117.5.1006. PMID 14570550.
- Garland Jr, Theodore; Morgan, Martin T; Swallow, John G; Rhodes, Justin S; Girard, Isabelle; Belter, Jason G; Carter, Patrick A (2002). "Evolution of a Small-Muscle Polymorphism in Lines of House Mice Selected for High Activity Levels". Evolution. 56 (6): 1267–75. doi:10.1554/0014-3820(2002)056[1267:EOASMP]2.0.CO;2. PMID 12144025.
- Gallaugher, P.E.; Thorarensen, H; Kiessling, A; Farrell, A.P. (2001). "Effects of high intensity exercise training on cardiovascular function, oxygen uptake, internal oxygen transport and osmotic balance in chinook salmon (Oncorhynchus tshawytscha) during critical speed swimming". The Journal of Experimental Biology. 204 (Pt 16): 2861–72. PMID 11683441.
- Palstra, A.P.; Mes, D; Kusters, K; Roques, J.A.; Flik, G; Kloet, K; Blonk, R.J. (2015). "Forced sustained swimming exercise at optimal speed enhances growth of juvenile yellowtail kingfish (Seriola lalandi)". Frontiers in Physiology. 5: 506. doi:10.3389/fphys.2014.00506. PMC 4287099. PMID 25620933.
- Magnoni, L.J.; Crespo, D; Ibarz, A; Blasco, J; Fernández-Borràs, J; Planas, J.V. (2013). "Effects of sustained swimming on the red and white muscle transcriptome of rainbow trout (Oncorhynchus mykiss) fed a carbohydrate-rich diet". Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 166 (3): 510–21. doi:10.1016/j.cbpa.2013.08.005. PMID 23968867.
- Owerkowicz T, Baudinette RV (2008). "Exercise training enhances aerobic capacity in juvenile estuarine crocodiles (Crocodylus porosus)". Comparative Biochemistry and Physiology A. 150 (2): 211–16. doi:10.1016/j.cbpa.2008.04.594. PMID 18504156.
- Eme, J; Owerkowicz, T; Gwalthney, J; Blank, J.M.; Rourke, B.C.; Hicks, J.W. (2009). "Exhaustive exercise training enhances aerobic capacity in American alligator (Alligator mississippiensis)". Journal of Comparative Physiology B. 179 (8): 921–31. doi:10.1007/s00360-009-0374-0. PMC 2768110. PMID 19533151.
- Butler, P.J.; Turner, D.L. (1988). "Effect of training on maximal oxygen uptake and aerobic capacity of locomotory muscles in tufted ducks, Aythya fuligula". The Journal of Physiology. 401: 347–59. doi:10.1113/jphysiol.1988.sp017166. PMC 1191853. PMID 3171990.
- Garland T, Else PL, Hulbert AJ, Tap P (1987). "Effects of endurance training and captivity on activity metabolism of lizards". Am. J. Physiol. 252 (3 Pt 2): R450–56. doi:10.1152/ajpregu.1987.252.3.R450. PMID 3826409. S2CID 8771310.
- Husak, J.F.; Keith, A.R.; Wittry, B.N. (2015). "Making Olympic lizards: The effects of specialised exercise training on performance". Journal of Experimental Biology. 218 (6): 899–906. doi:10.1242/jeb.114975. PMID 25617462.
External links
- Adult Compendium of Physical Activities – a website containing lists of Metabolic Equivalent of Task (MET) values for a number of physical activities, based upon PMID 8292105, 10993420 and 21681120
- Physical activity and the environment – guidance on the promotion and creation of physical environments that support increased levels of physical activity.
- MedLinePlus Topic on Exercise and Physical Fitness
- Science Daily's reference on physical exercise